Like many other people, the natural world has fascinated me from a very young age. I spent countless hours in my childhood drawing, reading about, and watching documentaries on animals such as tigers, brown bears, lions, various species of deer, buffalo, and so much more. I learned about the diets, physical attributes, and geographic distributions of most iconic animals. What had initially started as a captivation with individual animal species eventually morphed into an interest in animal communities and ecosystems such as taigas, tundras, African savannahs, Indian forests, and so on.
I admired every ecosystem for what it had to offer in terms of wildlife. However, I soon made an observation that I found rather odd from an ecological standpoint, and I had spent a while trying to find an explanation for it. It seemed to me that a disproportionate number of medium and large-sized animals live in Sub-Saharan Africa and secondarily in southern Eurasia, with the rest of the continents being rather impoverished in this regard. I did further research to confirm that this was indeed the case and not something I was imagining.
This was strange—it was not as if other parts of the world were lacking in biodiversity as a whole. South America, for example, contains the most species of any continent1. My sentiment about the unevenness of medium-large animal diversity was further solidified by taking family trips to various natural areas of the world. There could not have been a larger contrast between what I saw in terms of large animal abundance and diversity in Tanzania versus in Brazil, despite the fact that Brazil is itself extremely biodiverse. I wondered if perhaps there was something about Africa that had simply made it more conducive for the radiation of species of certain sizes than other continents.


But this confusion that had long plagued me was finally put to rest when I learned about the Late Quaternary (often synonymous with Late Pleistocene) extinctions. It turned out, to my surprise, that every continent besides Antarctica had once had its own set of plentiful and diverse megafauna (animals larger than 45 kg) but that these disappeared during the Late Pleistocene and parts of the Holocene2. Animals that were lost included iconic creatures such as mammoths and sabre-toothed cats among many others. I read about how the extinctions coincided with the arrival of anatomically modern humans to new landmasses where they encountered—and overhunted—animals that were not accustomed to them. Everything started to make sense.

As I started to dive deeply into paleontology over the course of the last few years, I was surprised to learn that the causes of the extinctions turned out to be much more controversial among both scientists and science enthusiasts than I had initially imagined. The most prominently invoked of the causes are the aforementioned human beings3 4 5, but also climate change6 7. The relative roles of humans and climate change are a matter of considerable debate, with experts and others who engage in the topic varying markedly in terms of how much importance they place on one versus the other. A recent review indicates that about a quarter of papers on the Late Quaternary extinctions place the blame on humans, another quarter on climate change, while the remainder either support mixed causes or take no stance8. This lack of a consensus may cause people to question the preeminence of humans in the extinctions.
However, this should not be the case—certainly not in the current year. While it is undeniable that climate change has been one of the main drivers of species-loss throughout geologic time, there is more than enough reason to believe that human activity was by far the more important piece of the extinction puzzle in the Late Quaternary. In fact, the evidence has pointed in this direction for quite a while. Still, there is a considerable amount of confusion that obscures what should be an obvious reality. Confusion which stems, partly, from people lacking a proper framework to understand the extinctions and how they fit into the broader paleoclimatic and palaeoecological context of the Quaternary and Cenozoic as a whole.
Therefore, my goal here is not solely to dump facts on the reader but to show them the right way to think about the topic. In this first article of a three-part series, I will point out the numerous flaws in climatic arguments, while the sequel will further expound on those flaws to make a case for why humans were the most important driving force as well as why this makes perfect sense. The third and last article will take a critical look at scientific literature that overemphasizes the climatic dimension to demonstrate why we should be more skeptical of their viewpoint, and to explore how bias may be distorting the debate. This series will be detailed and thorough; ideally, I will have made my points well enough that I will not need to write any more articles dedicated to the topic.
In the interest of nuance and precision, I will use the term “climate-heavy” to encompass theories that allege that climate was the dominant cause of the LQE, as well as those that assign climate a roughly equal or synergistic role alongside humans. Both of these perspectives, as I will explain, are unsupported by available evidence. They stand in opposition to human-centered explanations, which ascribe far greater significance to human impacts and are far more plausible models of extinction.
As there is a lot of information here, it is fine if you as the reader want to jump around to sections that interest you the most. However, I do encourage not skipping the next part on the background as it does contain some important information.
Background and Clarification on Terms
The Late Quaternary Extinctions are not clearly defined but they are usually used to describe a particular pattern of biodiversity loss that took place prior to modernity and industrialization in human societies. The term “Late Quaternary Extinctions” is often used interchangeably with “Late Pleistocene Extinctions but the latter is less problematic as extinctions in many cases stretched well past the end of the Pleistocene. Extinction waves on islands could be especially recent while even iconic “ice age” species such as woolly mammoths and the Irish elk Megaloceros lingered on in a few areas until the middle of the Holocene. The Late Quaternary period encompasses both the Late Pleistocene and Holocene. Therefore, the term “Late Quaternary Extinctions” (LQE from here on) is more suitable to describe these losses than “Late Pleistocene Extinctions” as it encompasses pre-modern extinctions in both epochs.
While extinctions are a fact of life on Earth, those in the Late Quaternary were unique in that megafauna (animals weighing over 45 kg) were lost at numbers greatly disproportionate to their share of the total faunal diversity2 4 5. The vast majority of the world’s fauna are not megafauna, but the LQE caused the majority of the world’s terrestrial megafauna to go extinct while small animal diversity along with marine animal and plant diversity remained largely intact despite some losses.
Further, amongst megafauna, the intensity of extinction increased with size with the largest species disappearing disproportionately. Megaherbivores, animals weighing over 1000kg, were present across all continents except Antarctica for much of the Cenozoic but by the late Holocene could only be found in Sub-Saharan Africa and southern Eurasia5 9. Indeed, the losses were not consistent across geography-islands experienced the highest rates while the African continent experienced rather low extinctions, with the exception of North Africa which is a complex outlier.
In 1974, an American geoscientist named Paul Martin proposed an intriguing yet controversial hypothesis that North America’s extinct megafauna were wiped out by the early human settlers, to whom the native megafauna had few defensive traits against3 10. This was inspired by observations about rapid extinctions following human arrival in islands like New Zealand. He called this the “overkill” hypothesis, and this model was eventually applied not only to North America but also South America and Australia. Eurasia had comparatively milder extinctions but proponents of anthropogenic causation argue that humans certainly had a role there as well, even if they acknowledge that climate may have been an important stressor.
Others were not exactly convinced, for one reason or another. Critics levy a number of arguments against the overkill hypothesis, noting things such as the rarity of evidence for human predation on extinct megafauna, the possibility that megafauna went extinct earlier than or much later than human arrival, and the human population allegedly being too small to have a major effect on the megafauna6 7. Many of those who reject predominantly anthropogenic explanations tend to favor a primary role for climate change6 11 12, with arguments ranging from the climate becoming too wet, too dry, too warm, or too cold for animals depending on the region. Others yet support mixed human-climate models13 14.
Some have argued for other causes for extinction as well but these lack support. The notion that an extraterrestrial impact triggered the Younger Dryas and wiped out North America’s megafauna has been firmly rejected by the scientific community15, while a hyper-disease remains implausible16. Many people, especially archeologists, hold the view that a “combination of several factors” may have led to the extinction of megafauna17. Upon further inspection, however, there are virtually no relevant factors that are not essentially just mechanisms of human and/or climate impacts anyway; for example, it is hard to see how malnutrition, habitat loss, and increased competition occurred independently of the aforementioned broad causes. When all is said and done, we ultimately end up back in square one weighing humans and climate against each other.
Still, experts do not necessarily attribute the extinctions solely to humans or solely to climate. Different scientists vary substantially in terms of how much emphasis they place on one versus the other so it is unhelpful to use vague terms such as “mixed causes” without being more specific. For example, an extinction event that can be 90% attributed to humans and 10% to climate is evidently very different from one that can be 90% attributed to climate and 10% to humans, so we need to be clear with what cause we are emphasizing. As stated earlier, I will be arguing for a mostly (but not exclusively) human-driven extinction event for the global LQE.
That said, the term “overkill” and especially its rapid “blitzkrieg” variant have certain problematic connotations and are often criticized as being too simplistic. I will therefore avoid using them-it is fully possible to argue for predominantly anthropogenically-driven extinctions without necessarily positing overhunting as the sole mechanism or claiming that extinctions were always extremely rapid and wave-like.
Additionally, it is important to point out that terms like “contribute” and “play a role” are vague and can cause confusion if not clearly defined. To say that a particular factor “contributed” to extinction may imply either that it was indispensable to the species’ disappearance or that it merely hastened an outcome that was already inevitable (due to other forces). This distinction matters: most of us interested in this debate are more concerned with whether extinct megafauna could have survived into the modern era absent a certain factor rather than with whether they might have lasted a bit longer.
Thus, framing causes in relation to final extinction is most meaningful. A useful guiding question with regard to the role of climate might be “was climate change really essential to the extinction of this particular taxon, or did it merely (at best) affect its precise timing?”. The same question should be asked with regard to the human role.
Having gotten these important details out of the way, we can move on to the substance of the article: discussing climate change, how it fits into paleoecology, and what we can learn about its potential role in the extinctions.
Climate and Extinction in Geologic Time
A good place to start when it comes to deciphering the Late Quaternary extinctions is evaluating the effects of climate on Earth’s ecosystems. Climate has been one of the most important drivers of extinction in Earth’s history. Plants and animals are adapted to a particular range of conditions called niches, and these can disappear if the climate changes in a detrimental way. If it becomes too hot, too cold, too dry, or too wet for an organism, extinction can result. And due to the complex nature of the Earth’s climate system, both climate change and extinctions resulting from it have been a fixture of Earth’s history since the beginning of life. Of the multiple mass extinction events in Earth’s history, many are known to have had a climatic cause which explains why climate has become a central suspect in the LQE.
Climatic swings during the Quaternary (spanning 2.58 million years ago to the present) were more intense than during earlier periods as it featured extensive glacial advances. The term “ice age” can mean different things depending on the context. They are often (including on this blog) used to refer specifically to glacial periods, but in the broadest and most formal/scientific sense, ice ages are synonymous with icehouse Earth periods when continental ice sheets are present, as opposed to greenhouse Earth periods when they are not present and glaciers only exist in high latitude mountains. The Late Cenozoic ice age started 34 million years ago with the formation of Antarctic ice sheets while the Quaternary glaciation, relevant to our discussion, commenced around 2.58 million years ago with the establishment of an ice sheet over Greenland. Using this broad, formal definition of “ice age”, we are still technically in one.
The Quaternary glaciation featured a large amplitude of change in temperature and ice mass, with periods being divided into glacials and interglacials. Glacial periods are colder and feature prominent ice sheets outside Greenland and Antarctica whereas in interglacials (like the one the Earth is currently in), the climate is warmer and the ice sheets are restricted to the poles. However, there is still considerable complexity as glacial periods are lengthy and involve a huge range of internal variation in ice sheet volume and climate. For example, cold and warm periods within glacials are known as stadials and interstadials respectively. Glacial periods also feature a few thousand years towards the end of a glacial cycle when ice volume reaches its peak known as glacial maxima. The midpoint of the most recent one, the Last Glacial Maximum (LGM), is considered to have been roughly 21 thousand years ago.
The profound and often rapid shifts in climate taking place during the Quaternary unsurprisingly led to major shifts in the ranges of plants and animal species, as well as the compositions of ecological communities18 19. Both extinctions and speciations (caused by populations becoming separated from each other and diverging) involving specific taxa have been attributed to these dramatic shifts but, shockingly, there is no strong evidence that climate-driven environmental changes during the Pleistocene led to significantly higher rates of extinction or speciation overall compared to previous periods despite the fact that the intensity of said changes had increased18 19 20. This is relevant to our next section when we discuss the Late Quaternary specifically, and the staggering loss of biodiversity amongst primarily (but not exclusively) megafaunal species.
The Late Quaternary Exception
Most of the Quaternary and Cenozoic in general involved a slow trickle of extinctions spread out over a large swathe of time—otherwise known as background extinctions4 18 19. Megafauna did not go extinct at unusually high rates compared to small animals. Very often, competition with other species was involved alongside climate change, and in many cases, these species were succeeded by others that directly descended from them. For example, the steppe mammoth in Eurasia was replaced by the woolly mammoth. The broad-fronted moose was replaced by the modern moose. The Armbruster’s wolf was replaced by the dire wolf, and so on. Extinctions in a given climate cycle were usually at the species or genera level and never involved the collapse of large animal communities across whole continents.
This is different from what happened starting around 50 thousand years ago. Very different. The extinctions were highly size-biased, and total loss of megafaunal genera during this period could approach 90% in places like Australia21. Megaherbivores, once present on all continents except Antarctica, disappeared across most of the world and can now only be found in southern Eurasia and Sub-Saharan Africa. At least at the continental scale, there is no strong pattern in terms of dietary or habitat specialization that can be gleaned from which species survived and which did not, as virtually all animals present during this period displayed some degree of ecological breadth5. Ecosystems were drastically and permanently changed with many niches still being vacant9 22.
Extinction waves usually occurred within short spaces of time and went far beyond the genera level. In some continents, entire orders and families of animal were wiped out despite thriving just a few thousand years prior23 24; for example, proboscideans, horses, glyptodonts, and ground sloths in the Americas. These were all taxa which had been continuously present in the New World since the Miocene or even Oligocene, long before the onset of Quaternary glaciations. Each of these groupings contained numerous species each with their own unique ecologies, diets, and geographic ranges but vanished regardless. And all of this happened despite small animal, marine, and plant diversity remaining fairly intact.

Although this was not technically a mass-extinction event, it was still very unprecedented due to its peculiarity. To describe the Late Quaternary extinctions as anything other than bizarre and catastrophic would not do them justice. They cannot be spoken of casually. But this also means they cannot be ascribed to a casual cause, which leads us to the problem that inevitably results when trying to pin all or most of the blame on climate change: the climatic fluctuations taking place during the Late Quaternary were not unusual. The graph below showing CO2, temperature, and dust concentration changes over the past 400,000 years based on ice core data from Antarctica illustrates this well.
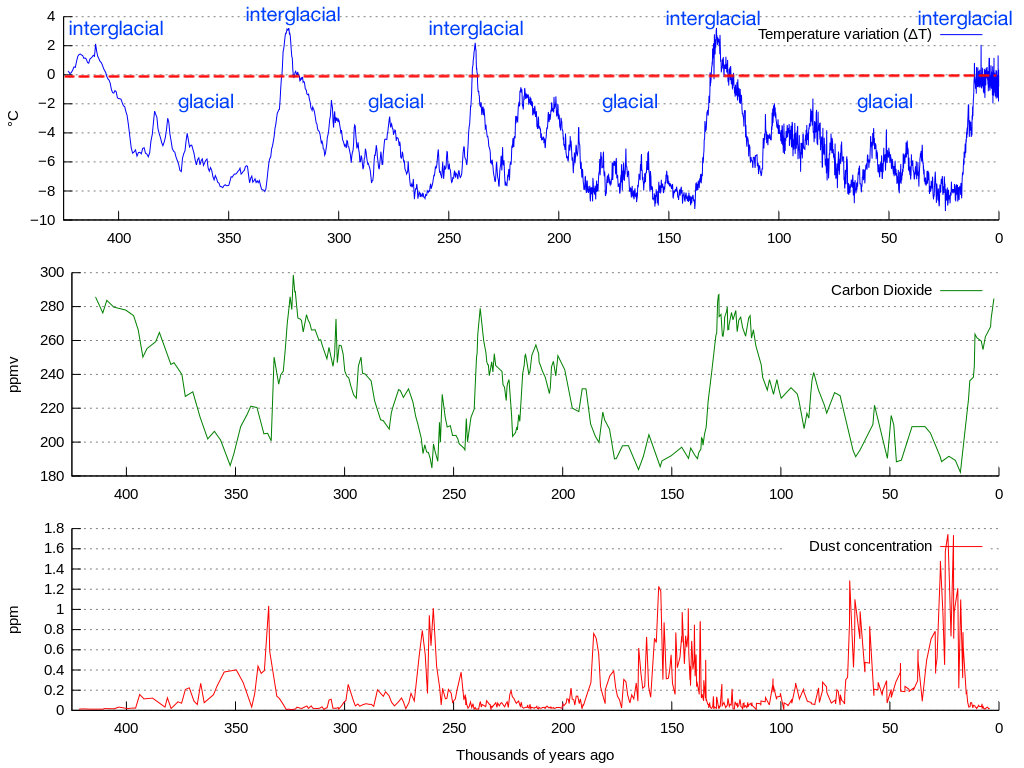
To be clear, there are some differences between various Quaternary interglacials and glacials, as there should be given how complex the Earth’s climate system is. But as far as individual climate shifts that occurred in the past 50 thousand years, they each have analogues elsewhere in the Quaternary when widespread megafaunal collapse did not occur. For example, the Younger Dryas cooling has received much attention as it coincides with extinctions in North America25 26 but it appears to not be a one-time event as a similar occurrence, indicated by Chinese stalagmites, took place around 245 thousand years ago at the end of the third to last glacial termination27. Previous interglacials were as warm or warmer than today28, so warming at the end of the last glacial could not have been too extreme for megafauna either.
Moreover, there is considerable internal variation within glacial periods, where temperatures could fluctuate between near-interglacial conditions during interstadials to deep glacial cold during stadials within a matter of decades or centuries29. The graph below from a paleoclimate paper30 shows the frequently chaotic nature of climate shifts over the past 130 thousand years, not just during glacial-interglacial transitions.
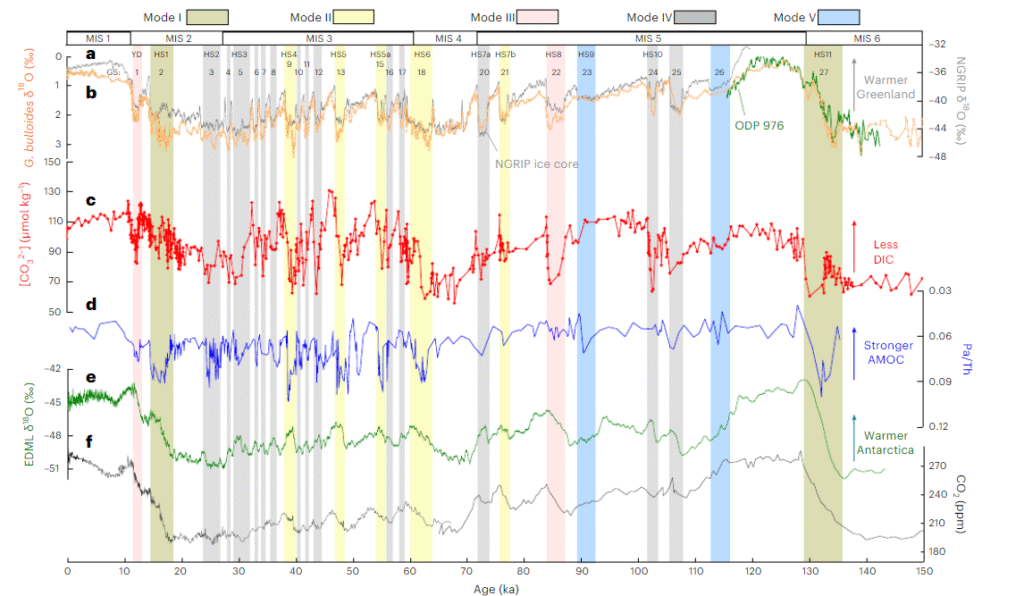
So when we talk about animal communities collapsing during this timeframe, we need to understand that they survived not only previous glacial-interglacial transitions, but also the turbulence within glacial periods. That makes it much harder to justify framing, for example, the climate change in the Americas between 13 and 11 thousand years ago when extinctions occurred there23 24 as an exceptional stressor for them. Then we also have the issue of the fact that extinctions in Sahul took place much earlier, between 50 and 40 thousand years ago, while in Eurasia they were spread out across the past 50 thousand years31. Island extinctions meanwhile took place during the relatively stable Holocene.
In keeping with long-term trends, it is inevitable that over the past few tens of millennia, some animals would have gone extinct from climate change just as they always had. A portion of these would have been megafauna. To claim that climate had no impact on megafaunal extinctions during the Late Quaternary would therefore be wrong. At the same time, megafaunal losses in the Late Quaternary were so unique and intense compared to those that took place earlier in the Cenozoic that a purely or mostly climatic explanation seems hard to justify as they go far beyond the normal rate of background extinctions.
But correctly pointing out that the climate was always changing and that animal communities survived previous climate cycles is still just scratching the surface—we need a nuanced understanding of climate change to elucidate why they did in order to truly grasp the peculiar nature of extinctions during the Late Pleistocene and part of the Holocene.
Why Climate Fails as a Dominant Cause
In our modern world, anthropogenic climate change poses a real threat to humans and animals. It has therefore become a common view among certain segments of society that climate change is inherently bad for living things, and this view has colored perceptions of the LQE. In fact, a few papers that argue for climate-heavy extinction causes directly reference modern anthropogenic climate change in order to demonstrate real world utility for their research7 32 33.
But this attempt to link past and modern climate change ignores that the Earth of old was dramatically different from the one we currently occupy; as opposed to the ubiquitous agricultural lands and settlements that fracture animal habitats into islands today, an endless wilderness covered the planet in prehistoric times. In that world, the only barriers to plant and animal (including human) dispersal were natural, geographical ones rather than man-made ones. That reduces the power of comparison between the effects of modern and prehistoric climate changes drastically as dispersal ability, which is critical for organisms when it comes to surviving climate shifts, was far greater back then.
Further, to plainly state that climate change is bad for plants and animals as a whole would be quite misleading. Rather, it is more accurate to say that climate change *can* be bad for plants or animals depending on the type of climate change involved as well as the species being affected. For example, the climate in Beringia at the end of the last glacial period shifted from a drier one to a wetter one and this was not good for woolly mammoths and horses, but moose were able to expand their range into the new shrub and taiga covered landscape and expand deep into North America34. Moose certainly would not complain about that climate change.
The LGM, which lasted roughly from 25 to 19 thousand years ago, was actually a relatively stable period. Despite this stability, animals large and small frequently did better following the LGM than during it. Post-LGM warming benefitted a number of North American animals such as black bears and shrews35 as well as several species of Holarctic birds (even ones adapted to open habitat)36. In the seas, both killer whales—which experienced a major LGM bottleneck—and Antarctic Fur Seals followed a similar pattern37 38. Even northern populations of woolly mammoths declined during the LGM and then rebounded shortly afterwards39.
The trends among Antarctic Fur Seals and northern woolly mammoths are especially revealing—it could very well be that the LGM was so icy, so windy, and so low in net primary productivity that it was not optimal even for species (or certain populations of said species) typically associated with frigid conditions. It is therefore no surprise that the warming after the LGM is frequently described as a “climatic amelioration”. But there is a critical lesson here: it is not solely climate change in and of itself that is important for organisms, but also what the climate is changing from and to.
It is well-known that species respond to climate change individualistically, and this is true even for members of the same ecosystems19 40. The Quaternary world featured a very wide selection of habitats and climate types, and animals differed widely in terms of their ecological preferences and needs. A change that was good for one species may have been bad for another, and vice versa. Moreover, there is strong evidence that Quaternary megafauna were flexible in terms of diet and habitat preferences to one degree or another5—they had to be in order to survive the constant climate shifts.
Meanwhile, climate change itself is deeply complex. Rather than having uniform and sweeping effects, a particular climate event can cause wetter conditions in one area and drier conditions in another, or warmer conditions in one area and cooler ones in another. Some places are more stable climatically than others. The start of the Younger Dryas in North America coincides roughly with the start of extinctions there, but changes were anything but homogenous with even nearby locales differing in terms of how they responded to it: some became cooler and wetter while others became cooler and drier, and others yet changed little or became warmer41. The map below illustrates this well (ironically, it was compiled by David Meltzer, possibly the most prominent opponent of overkill in North America).
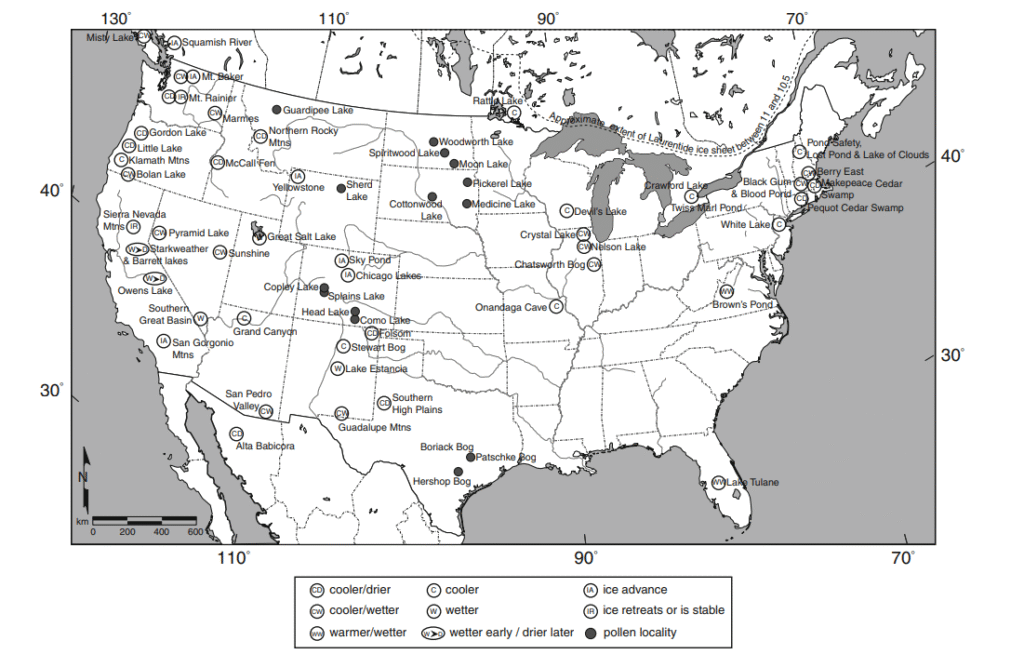
This geographical variation in the effects of climate has important ramifications. It means that suitable habitat for a given species is likely to persist in one place or the other, even if it diminishes in another. For example, the American southwest experienced intense aridification during the Bølling–Allerød interstadial which negatively impacted many megafauna in areas such as southern California42. However, the jet stream simultaneously shifted north and moistened the Pacific northwest43, so suitable habitat should have existed in the western United States anyway. The eastern United States, meanwhile, has maintained biodiversity as warm and wet conditions persisted in the southeast during cold Heinrich events44.
In other words, the complexity of Quaternary animal ecology must be stacked on top of the nuances of climate shifts to understand why animal communities-including the extinct megafauna-were generally resilient. It explains why throughout most of the Quaternary and Cenozoic as a whole, animals did not all hit the bucket simultaneously during brief pulses of climate change. Further, this resilience and adaptability combines with continuous speciation to explain why megafaunal diversity remained high and why exceptionally large-bodied animals persisted throughout the Cenozoic right up until a few tens of thousands of years ago.
The effects of Quaternary climate changes on plants and animals should therefore be viewed as a perpetual shuffle; some species go extinct while new ones emerge, and the ranges and abundances of some species contract while others expand. This makes the LQE difficult to square with a climate-dominant causation, because extinction pulses causing the collapse of whole communities of megafauna often occurred rapidly-in under a few thousand years in some cases.
There is more to explore with regard to climate and extinction, which is the inconvenient reality that megafaunal communities thrived much longer on islands than on continents, as well as evidence for a number of species benefitting from climate changes (such as glacial-interglacial transitions) that were supposedly fatal for extinct megafauna.
Island Survival and Demographic Trends
There is considerable fossil and genomic evidence for demographic resilience and even expansion which defies climatic narratives. We will first start by talking about islands: it is well-known that megafaunal communities survived on islands much longer than on adjacent continents. Examples include woolly mammoths on St. Paul and Wrangel islands, ground sloths in the Caribbean, and Moa in New Zealand which all survived into the mid to late Holocene31. Additionally, genetic analyses of the animals living on islands reveal evidence of demographic stability. There is no reason for this to be the case under a hypothetical world where animal communities as a whole were highly sensitive every major climate shift.
For example, woolly mammoths on Wrangel Island only went extinct around 4 thousand years ago, in stark contrast to their counterparts on the climatically identical Siberian mainland which went extinct close to the start of the Holocene. It was frequently speculated that these last mammoths may have gone extinct from inbreeding, which caused some sort of genomic meltdown45. But a newer study revealed that the mammoths, while inbred, were healthy as they had purged many harmful mutations from their gene pool46. It is not clear exactly how the mammoths on this remote island perished, but their extended survival on a tiny island well past the glacial-interglacial transition reveals how resilient megafauna can be in the absence of humans.
Then there are the Moa. These animals were wiped out by the Maori only a few centuries ago. Amazingly, genetic evidence indicates that their effective population size had been stable for the past 40,000 years, meaning they were resilient to the full range of climatic variability47. Indeed, the ecology of New Zealand had remained largely the same for the past 100,000 years right up until human settlement48. Moreover, if this was true for New Zealand’s megafauna, it should also have been true for the megafauna of southeastern Australia and Tasmania since the climate characteristics and forcings between their respective regions are very similar (rainfall patterns dominated by southern hemisphere westerlies), and yet those perished tens of thousands of years earlier. The only explanation is differential dates in the settlement of man.

There is no logical reason for islands to have retained megafauna for longer and/or for insular megafauna to have had greater demographic stability than their mainland counterparts. It is well-known that insular megafauna are more likely to go extinct than their mainland counterparts. The small sizes of islands means animals are more vulnerable to sea level rise, unlike on mainlands where they can retreat endlessly towards continental interior. Smaller sizes of islands also results in a lower total diversity of climate zones and habitats, which can mean fewer or smaller refugia for animals during times of climatic deterioration.
As for mainlands, there is evidence that glacial-interglacial transitions are not necessarily times of stress and contraction but can result in demographic and range expansions among animals. It is not surprising that the most cold-adapted animals would experience a reduction in habitat with intense warming, as occurred with reindeer and woolly mammoths49 50, but the vast majority of animals globally were and still are either temperate or tropical/subtropical. An expectation of stability or population/geographic growth seems logical for the majority of animal species, but this has been obscured by the fact that disproportionate focus has been given to extinct animals living in boreal zones.
As mentioned earlier in this article, black bears as well as many small mammals in North America expanded demographically between the Last Glacial Maximum and the Holocene, with northern populations expanding into areas that opened up with retreating ice35. A landmark study which looked at the demographic histories of extant animals on the continents found trends of population stability prior to the expansion of anatomically modern humans out of Africa, meaning they were largely resilient to glacial-interglacial changes which occurred several times during the Pleistocene51.
Pleistocene animals were no less ecologically flexible as a whole than their counterparts who survived into recent times, so we have reason to expect that similar trends of interglacial stability or even expansion applied to them. Mastodons and Jefferson’s ground sloths, which during earlier interglacials had reached far into eastern Beringia (Yukon and Alaska), demonstrate this trend52 53. The short-faced bear Arctodus simus also occupied these northern territories prior to the LGM54, so we could expect that it, along with mastodons and Jefferson’s ground sloths, should have recolonized the area following post-LGM warming and opening up of the ice-free corridor. Yet, all of these disappeared in the midst of deglaciation.
What we have here is 1) Evidence of insular animal communities thriving well past periods of major climatic change such as the last glacial-interglacial transition and 2) Evidence that many mainland animals benefitted from glacial-interglacial transitions via range expansion. Neither of these points is compatible with the idea that animal communities as a whole were highly sensitive to periods of strong climate change.
Summary and Sequel
Despite there being hundreds of blog articles and scientific papers on the Late Quaternary extinctions, coming up with a good framework to contextualize and understand the extinctions can be challenging. Providing that framework is the goal of this series. To summarize everything in this first article in the most effective way possible:
- Climate change has been a major driver of extinctions throughout Earth’s history, but previous high amplitude/high velocity climate changes during the Quaternary are not known to have culminated in widespread extinctions, and previous extinctions were not nearly as size-biased as the Late Quaternary ones.
- Extinctions occurred in a largely trickle-like fashion throughout the Quaternary, rather than catastrophic events as in the Late Quaternary. This is because:
A) Animals as a whole had to adapt to the constantly shifting climates and environments of the Quaternary world, and hence evolved to became resilient to one degree or another.
B) Different animals have different ecologies and hence respond to climatic changes individualistically, reducing the likelihood of “one-size fits all” climatic deteriorations wiping out or putting grave stress on animal communities as a whole.
C) Climate change itself is intricate in terms of its effects because different regions in a given continent experience different degrees and directionalities in changes (hotter, colder, wetter, drier), so detrimental changes in one locale may not be reflect broad trends. - This makes the Late Quaternary Extinctions hard to square with a majority climatic cause, because whole continents were dealt heavy blows to megafaunal diversity, in many cases in very short periods of time.
- Demographic patterns call into question the sensitivity of animals as a whole to climate shifts; island megafauna survived much longer than their mainland counterparts while maintaining genetic stability, a trend mirrored by many mainland animals-extinct and extant-who thrived during glacial-interglacial transitions (even the last one in some cases).
So far, we have enough reason to rule out climate as the main or most important cause of megafaunal extinctions for the past 50 thousand years, but there is still more to say with regard to the role of climate versus humans. The next part of this series will look at how the evidence from biogeographic extinction patterns is especially damning to climate narratives, and then compare and contrast the potency of humans and climate change to establish why humans were, by far, the more important force.
Part 2: Biogeography and the Human Onslaught
References
- Raven, P. H., Gereau, R. E., Phillipson, P. B., Chatelain, C., Jenkins, C. N., & Ulloa, C. U. (2020). The distribution of biodiversity richness in the tropics. Science Advances, 6(37), eabc6228. https://doi.org/10.1126/sciadv.abc6228[↩]
- Koch, P. L., & Barnosky, A. D. (2006). Late Quaternary Extinctions: State of the Debate. Annual Review of Ecology, Evolution, and Systematics, 37(1), 215–250. https://doi.org/10.1146/annurev.ecolsys.34.011802.132415[↩][↩]
- Martin, P. S., & Klein, R. G. (1995). Quaternary extinctions : a prehistoric revolution. University Of Arizona Press.[↩][↩]
- Sandom, C., Faurby, S., Sandel, B., & Svenning, J.-C. (2014). Global late Quaternary megafauna extinctions linked to humans, not climate change. Proceedings of the Royal Society B: Biological Sciences, 281(1787), 20133254. https://doi.org/10.1098/rspb.2013.3254[↩][↩][↩]
- Svenning, J.-C., Lemoine, R. T., Bergman, J., Buitenwerf, R., Roux, E. L., Lundgren, E., Mungi, N., & Pedersen, R. Ø. (2024). The late-Quaternary megafauna extinctions: Patterns, causes, ecological consequences and implications for ecosystem management in the Anthropocene. Cambridge Prisms: Extinction, 2, e5. https://doi.org/10.1017/ext.2024.4[↩][↩][↩][↩][↩]
- Grayson, D. K., & Meltzer, D. J. (2003). A requiem for North American overkill. Journal of Archaeological Science, 30(5), 585–593. https://doi.org/10.1016/s0305-4403(02)00205-4[↩][↩][↩]
- Wroe, S., Field, J., & Grayson, D. (2006). Megafaunal extinction: climate, humans and assumptions. Trends in Ecology & Evolution, 21(2), 61–62. https://doi.org/10.1016/j.tree.2005.11.012[↩][↩][↩]
- Stewart, M., Peters, C., Ziegler, M. J., Carleton, W. C., Roberts, P., Boivin, N., & Groucutt, H. S. (2025). The state of the late Quaternary megafauna extinction debate: a systematic review and analysis. Frontiers in Mammal Science, 4. https://doi.org/10.3389/fmamm.2025.1678231[↩]
- Owen-Smith, N. (1987). Pleistocene extinctions: the pivotal role of megaherbivores. Paleobiology, 13(3), 351–362. https://doi.org/10.1017/s0094837300008927[↩][↩]
- Martin, P. S. (1973). The Discovery of America: The first Americans may have swept the Western Hemisphere and decimated its fauna within 1000 years. Science, 179(4077), 969–974. https://doi.org/10.1126/science.179.4077.969[↩]
- Meltzer, D. J. (2020). Overkill, glacial history, and the extinction of North America’s Ice Age megafauna. Proceedings of the National Academy of Sciences, 117(46), 28555–28563. https://doi.org/10.1073/pnas.2015032117[↩]
- Wroe, S., Field, J. H., Archer, M., Grayson, D. K., Price, G. J., Louys, J., Faith, J. T., Webb, G. E., Davidson, I., & Mooney, S. D. (2013). Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea). Proceedings of the National Academy of Sciences, 110(22), 8777–8781. https://doi.org/10.1073/pnas.1302698110[↩]
- Metcalf, J. L., Turney, C., Barnett, R., Martin, F., Bray, S. C., Vilstrup, J. T., Orlando, L., Salas-Gismondi, R., Loponte, D., Medina, M., De Nigris, M., Civalero, T., Fernández, P. M., Gasco, A., Duran, V., Seymour, K. L., Otaola, C., Gil, A., Paunero, R., & Prevosti, F. J. (2016). Synergistic roles of climate warming and human occupation in Patagonian megafaunal extinctions during the Last Deglaciation. Science Advances, 2(6), e1501682. https://doi.org/10.1126/sciadv.1501682[↩]
- Broughton, J. M., & Weitzel, E. M. (2018). Population reconstructions for humans and megafauna suggest mixed causes for North American Pleistocene extinctions. Nature Communications, 9(1). https://doi.org/10.1038/s41467-018-07897-1[↩]
- Holliday, V. T., Daulton, T. L., Bartlein, P. J., Boslough, M. B., Breslawski, R. P., Fisher, A., Jorgeson, I., Scott, A. C., Koeberl, C., Marlon, J. R., Severinghaus, J. P., Petaev, M. I., & Claeys, P. (2023). Comprehensive refutation of the Younger Dryas Impact Hypothesis (YDIH). Earth-Science Reviews, 104502–104502. https://doi.org/10.1016/j.earscirev.2023.104502[↩]
- Kathleen Lyons, S., Smith, F. A., Wagner, P. J., White, E. P., & Brown, J. H. (2004). Was a “hyperdisease” responsible for the late Pleistocene megafaunal extinction?. Ecology Letters, 7(9), 859–868. https://doi.org/10.1111/j.1461-0248.2004.00643.x[↩]
- Nagaoka, L., Rick, T., & Wolverton, S. (2018). The overkill model and its impact on environmental research. Ecology and Evolution, 8(19), 9683–9696. https://doi.org/10.1002/ece3.4393[↩]
- Bennett, K. D. (2004). Continuing the debate on the role of Quaternary environmental change for macroevolution. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359(1442), 295–303. https://doi.org/10.1098/rstb.2003.1395[↩][↩][↩]
- Blois, J. L., & Hadly, E. A. (2009). Mammalian Response to Cenozoic Climatic Change. Annual Review of Earth and Planetary Sciences, 37(1), 181–208. https://doi.org/10.1146/annurev.earth.031208.100055[↩][↩][↩][↩]
- Cohen, A. S., Du, A., Rowan, J., Yost, C. L., Billingsley, A. L., Campisano, C. J., Brown, E. T., Deino, A. L., Feibel, C. S., Grant, K., Kingston, J. D., Lupien, R. L., Muiruri, V., R. Bernhart Owen, Reed, K. E., Russell, J., & Stockhecke, M. (2022). Plio-Pleistocene environmental variability in Africa and its implications for mammalian evolution. Proceedings of the National Academy of Sciences of the United States of America, 119(16). https://doi.org/10.1073/pnas.2107393119[↩]
- Koch, P. L., & Barnosky, A. D. (2006). Late Quaternary Extinctions: State of the Debate. Annual Review of Ecology, Evolution, and Systematics, 37(1), 215–250. https://doi.org/10.1146/annurev.ecolsys.34.011802.132415[↩]
- Smith, F. A., Elliott Smith, E. A., Hedberg, C. P., Lyons, S. K., Pardi, M. I., & Tomé, C. P. (2023). After the mammoths: the ecological legacy of late Pleistocene megafauna extinctions. Cambridge Prisms: Extinction, 1(9), 1–55. https://doi.org/10.1017/ext.2023.6[↩]
- Faith, J. T., & Surovell, T. A. (2009). Synchronous extinction of North America’s Pleistocene mammals. Proceedings of the National Academy of Sciences, 106(49), 20641–20645. https://doi.org/10.1073/pnas.0908153106[↩][↩]
- Prates, L., & Perez, S. I. (2021). Late Pleistocene South American megafaunal extinctions associated with rise of Fishtail points and human population. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-22506-4[↩][↩]
- Fiedel, S., & Haynes, G. (2004). A premature burial: comments on Grayson and Meltzer’s “Requiem for overkill.” Journal of Archaeological Science, 31(1), 121–131. https://doi.org/10.1016/j.jas.2003.06.004[↩]
- Seersholm, F. V., Werndly, D. J., Grealy, A., Johnson, T., Keenan Early, E. M., Lundelius, E. L., Winsborough, B., Farr, G. E., Toomey, R., Hansen, A. J., Shapiro, B., Waters, M. R., McDonald, G., Linderholm, A., Stafford, T. W., & Bunce, M. (2020). Rapid Range Shifts and Megafaunal Extinctions Associated with Late Pleistocene Climate Change. Nature Communications, 11(1). https://doi.org/10.1038/s41467-020-16502-3[↩]
- Broecker, W. S., Denton, G. H., Edwards, R. L., Cheng, H., Alley, R. B., & Putnam, A. E. (2010). Putting the Younger Dryas cold event into context. Quaternary Science Reviews, 29(9-10), 1078–1081. https://doi.org/10.1016/j.quascirev.2010.02.019[↩]
- Coletti, A. J., DeConto, R. M., J. Brigham-Grette, & M. Melles. (2015). A GCM comparison of Pleistocene super-interglacial periods in relation to Lake El’gygytgyn, NE Arctic Russia. Climate of the Past, 11(7), 979–989. https://doi.org/10.5194/cp-11-979-2015[↩]
- Denton, G., Alley, R., Comer, G., & Broecker, W. (2005). The role of seasonality in abrupt climate change. Quaternary Science Reviews, 24(10-11), 1159–1182. https://doi.org/10.1016/j.quascirev.2004.12.002[↩]
- Yu, J., Anderson, R. F., Jin, Z. D., Ji, X., Thornalley, D. J. R., Wu, L., Thouveny, N., Cai, Y., Tan, L., Zhang, F., Menviel, L., Tian, J., Xie, X., Rohling, E. J., & McManus, J. F. (2023). Millennial atmospheric CO2 changes linked to ocean ventilation modes over past 150,000 years. Nature Geoscience, 16(12), 1166–1173. https://doi.org/10.1038/s41561-023-01297-x[↩]
- Stuart, A. J. (2014). Late Quaternary megafaunal extinctions on the continents: a short review. Geological Journal, 50(3), 338–363. https://doi.org/10.1002/gj.2633[↩][↩]
- Yansa, C. H., & Adams, K. M. (2012). Mastodons and Mammoths in the Great Lakes Region, USA and Canada: New Insights into their Diets as they Neared Extinction. Geography Compass, 6(4), 175–188. https://doi.org/10.1111/j.1749-8198.2012.00483.x[↩]
- Huntley, B., Allen, J. R. M., Collingham, Y. C., Hickler, T., Lister, A. M., Singarayer, J., Stuart, A. J., Sykes, M. T., & Valdes, P. J. (2013). Millennial Climatic Fluctuations Are Key to the Structure of Last Glacial Ecosystems. PLoS ONE, 8(4), e61963. https://doi.org/10.1371/journal.pone.0061963[↩]
- Monteath, A. J., Gaglioti, B. V., Edwards, M. E., & Froese, D. (2021). Late Pleistocene shrub expansion preceded megafauna turnover and extinctions in eastern Beringia. Proceedings of the National Academy of Sciences, 118(52). https://doi.org/10.1073/pnas.2107977118[↩]
- Lessa, E. P., Cook, J. A., & Patton, J. L. (2003). Genetic footprints of demographic expansion in North America, but not Amazonia, during the Late Quaternary. Proceedings of the National Academy of Sciences of the United States of America, 100(18), 10331–10334. https://doi.org/10.1073/pnas.1730921100[↩][↩]
- Miller, E. F., Green, R. E., Balmford, A., Maisano Delser, P., Beyer, R., Somveille, M., Leonardi, M., Amos, W., & Manica, A. (2021). Bayesian Skyline Plots disagree with range size changes based on Species Distribution Models for Holarctic birds. Molecular Ecology, 30(16), 3993–4004. https://doi.org/10.1111/mec.16032[↩]
- Moura, A. E., Janse van Rensburg, C., Pilot, M., Tehrani, A., Best, P. B., Thornton, M., Plön, S., de Bruyn, P. J. N., Worley, K. C., Gibbs, R. A., Dahlheim, M. E., & Hoelzel, A. R. (2014). Killer Whale Nuclear Genome and mtDNA Reveal Widespread Population Bottleneck during the Last Glacial Maximum. Molecular Biology and Evolution, 31(5), 1121–1131. https://doi.org/10.1093/molbev/msu058[↩]
- Cleary, A. C., Hoffman, J. I., Jaume Forcada, Lydersen, C., Lowther, A. D., & Kovacs, K. M. (2021). 50,000 years of ice and seals: Impacts of the Last Glacial Maximum on Antarctic fur seals. Ecology and Evolution, 11(20), 14003–14011. https://doi.org/10.1002/ece3.8104[↩]
- MacDonald, G. M., Beilman, D. W., Kuzmin, Y. V., Orlova, L. A., Kremenetski, K. V., Shapiro, B., Wayne, R. K., & Van Valkenburgh, B. (2012). Pattern of extinction of the woolly mammoth in Beringia. Nature Communications, 3(1). https://doi.org/10.1038/ncomms1881[↩]
- Graham, R. W., Lundelius, E. L., Graham, M. A., Schroeder, E. K., Toomey, R. S., Anderson, E., Barnosky, A. D., Burns, J. A., Churcher, C. S., Grayson, D. K., Guthrie, R. D., Harington, C. R., Jefferson, G. T., Martin, L. D., McDonald, H. G., Morlan, R. E., Semken, H. A., Webb, S. D., Werdelin, L., & Wilson, M. C. (1996). Spatial Response of Mammals to Late Quaternary Environmental Fluctuations. Science, 272(5268), 1601–1606. https://doi.org/10.1126/science.272.5268.1601[↩]
- Meltzer, D. J., & Holliday, V. T. (2010). Would North American Paleoindians have Noticed Younger Dryas Age Climate Changes? Journal of World Prehistory, 23(1), 1–41. https://doi.org/10.1007/s10963-009-9032-4[↩]
- F. Robin O’Keefe, Dunn, R. E., Weitzel, E. M., Waters, M. R., Martinez, L., Binder, W. J., Southon, J., Cohen, J. E., Meachen, J., Larisa R.G. DeSantis, Kirby, M. E., Ghezzo, E., Brenner-Coltrain, J., Fuller, B. T., Farrell, A. B., Takeuchi, G. T., MacDonald, G. M., Davis, E. B., & Lindsey, E. (2023). Pre–Younger Dryas megafaunal extirpation at Rancho La Brea linked to fire-driven state shift. Science, 381(6659). https://doi.org/10.1126/science.abo3594[↩]
- Lora, J. M., Mitchell, J. L., & Tripati, A. E. (2016). Abrupt reorganization of North Pacific and western North American climate during the last deglaciation. Geophysical Research Letters, 43(22). https://doi.org/10.1002/2016gl071244[↩]
- Fastovich, D., Radeloff, V. C., Zuckerberg, B., & Williams, J. W. (2024). Legacies of millennial-scale climate oscillations in contemporary biodiversity in eastern North America. Philosophical Transactions of the Royal Society B Biological Sciences, 379(1902). https://doi.org/10.1098/rstb.2023.0012[↩]
- Rogers, R. L., & Slatkin, M. (2017). Excess of genomic defects in a woolly mammoth on Wrangel Island. PLOS Genetics, 13(3), e1006601. https://doi.org/10.1371/journal.pgen.1006601[↩]
- Dehasque, M., Morales, H. E., Díez-del-Molino, D., Patrícia Pečnerová, J. Camilo Chacón-Duque, Foteini Kanellidou, Muller, H., Plotnikov, V., Protopopov, A., Tikhonov, A., Pavel Nikolskiy, Danilov, G. K., Maddalena Giannì, Laura, Higham, T., Heintzman, P. D., Nikolay Oskolkov, Gilbert, T. P., Anders Götherström, & Tom. (2024). Temporal dynamics of woolly mammoth genome erosion prior to extinction. Cell, 187(14). https://doi.org/10.1016/j.cell.2024.05.033[↩]
- Rawlence, N. J., Metcalf, J. L., Wood, J. R., Worthy, T. H., Austin, J. J., & Cooper, A. (2012). The effect of climate and environmental change on the megafaunal moa of New Zealand in the absence of humans. Quaternary Science Reviews, 50, 141–153. https://doi.org/10.1016/j.quascirev.2012.07.004[↩]
- Holdaway, R. N., Worthy, T. H., & Tennyson, A. J. D. (2001). A working list of breeding bird species of the New Zealand region at first human contact. New Zealand Journal of Zoology, 28(2), 119–187. https://doi.org/10.1080/03014223.2001.9518262[↩]
- Flagstad, Ø., & Røed, K. H. (2003). Refugial Origins of Reindeer (*Rangifer tarandus* L.) Inferred from Mitochondrial DNA Sequences. Evolution, 57(3), 658–670. https://doi.org/10.1111/j.0014-3820.2003.tb01557.x[↩]
- Palkopoulou, E., Dalen, L., Lister, A. M., Vartanyan, S., Sablin, M., Sher, A., Edmark, V. N., Brandstrom, M. D., Germonpre, M., Barnes, I., & Thomas, J. A. (2013). Holarctic genetic structure and range dynamics in the woolly mammoth. Proceedings of the Royal Society B: Biological Sciences, 280(1770), 20131910–20131910. https://doi.org/10.1098/rspb.2013.1910[↩]
- Bergman, J., Pedersen, R. Ø., Lundgren, E. J., Lemoine, R. T., Monsarrat, S., Pearce, E. A., Schierup, M. H., & Svenning, J.-C. (2023). Worldwide Late Pleistocene and Early Holocene population declines in extant megafauna are associated with Homo sapiens expansion rather than climate change. Nature Communications, 14(1), 7679. https://doi.org/10.1038/s41467-023-43426-5[↩]
- Zazula, G. D., MacPhee, R. D. E., Metcalfe, J. Z., Reyes, A. V., Brock, F., Druckenmiller, P. S., Groves, P., Harington, C. R., Hodgins, G. W. L., Kunz, M. L., Longstaffe, F. J., Mann, D. H., McDonald, H. G., Nalawade-Chavan, S., & Southon, J. R. (2014). American mastodon extirpation in the Arctic and Subarctic predates human colonization and terminal Pleistocene climate change. Proceedings of the National Academy of Sciences, 111(52), 18460–18465. https://doi.org/10.1073/pnas.1416072111[↩]
- G, M. H., R, H. C., & de Iuliis, G. (2000). The Ground Sloth Megalonyx from Pleistocene Deposits of the Old Crow Basin, Yukon, Canada. Arctic, 53(3), 213–220. JSTOR. https://doi.org/10.2307/40511905[↩]
- Salis, A. T., Bray, S. C. E., Lee, M. S. Y., Heiniger, H., Barnett, R., Burns, J. A., Doronichev, V., Fedje, D., Golovanova, L., Harington, C. R., Hockett, B., Kosintsev, P., Lai, X., Mackie, Q., Vasiliev, S., Weinstock, J., Yamaguchi, N., Meachen, J. A., Cooper, A., & Mitchell, K. J. (2021). Lions and brown bears colonized North America in multiple synchronous waves of dispersal across the Bering Land Bridge. Molecular Ecology. https://doi.org/10.1111/mec.16267[↩]
