
Nowhere is more central to the subject of the Late Pleistocene extinctions than the Americas. It was based on the staggering losses of megafauna here that an American geoscientist, Paul Martin, proposed his theory that the first humans to enter the New World were responsible for the elimination of most large-bodied species through overhunting, also known as “overkill”1.
His idea has been strongly contested of course, with others arguing that it was instead climate change which doomed the animals2 or at least contributed in a substantial way alongside humans3 4. Others yet argue for the importance of indirect effects of human activity on ecosystems5 6 7, while a few promote unsupported theories like a comet impact or hyper-disease8 9. The debate has continued for decades and it is unlikely a consensus will be reached any time soon.
This series will be divided into two parts. This is the first, wherein I meticulously discuss the inadequacy of climatic explanations for the extinctions, and then lay out why a primarily human causation makes more sense. The sequel will delve into what we know about human arrival, the evidence for human predation on megafauna, and examine the details of how extinctions may have unfolded as people spread through the New World.
It may seem odd to tackle the extinctions in both North and South America in just two posts, and it is certainly true that extinction dynamics in individual regions and ecosystems within both continents contain enough detail and complexity to warrant articles of their own. However, the Late Pleistocene extinction event is a topic which continues to create great confusion among both researchers and prehistory-enthusiasts alike, and one reason for this confusion is because many struggle to filter out irrelevant details and capture the big picture. As North and South America share a lot in terms of human history but little in terms of climate, discussing extinctions on both continents in the context of each other is absolutely necessary to tease out the relative roles of humans vs. climate.
Before we get started, we need to set up more context first.
Background and Challenges
Towards the very end of the Pleistocene, the large majority of American megafaunal genera-72% in North America and 83% in South America-went extinct10. The extinctions in the Americas have a lot in common with those in Australia, another focal point in the debate about overkill. In both cases, megafauna with no prior exposure to hominins went extinct soon after human arrival. In both cases, the typical suspects are climate and humans. There are important differences, however.
- The extinctions in the Americas were far more recent, resulting in a far better fossil record with more remains and better dating on those remains. This makes pinpointing when various megafauna disappeared easier, although not without difficulty.
- Whereas in Australia, there is only one site alleged to indicate a much earlier date for human entry than traditional estimates, in North and South America there are many. This has made it hard to determine precisely when humans arrived in the Americas.
- While claims about major climate change during the extinction window in Australia are controversial, there is no disputing that a dramatic shift was taking place when American megafauna died off.
- In Australia, there is no agreed-upon direct evidence for human predation on megafauna, such as kill sites, unlike in the Americas where there is plenty.
In my post on Late Quaternary Australian extinctions, I showed that narrowing down the timeframe for extinction and refuting claims about concurrent climate change are enough to attribute responsibility to humans. In the Americas by contrast, the undeniable climate shifts coinciding with human settlement complicate causation. The presence of climate change does not rule out a primarily human-driven extinction, nor does evidence of human predation negate a mainly climatic cause. Assuming both contributed roughly equally, on the other hand, risks falling into the middle-ground fallacy. A different approach is needed.
Making matters more complex is the debate over whether humans drove extinction through simple overhunting of each species(classic overkill), trophic disruption7, or whether the human use of fire and/or selective elimination of keystone species drastically altered environments leading to further extinctions5 6 . For the sake of simplicity, I will use “environmental change” here to refer to the kinds of broad biome changes primarily brought on by natural forces like climate, and then address the finer details of human impact on environments in the next part.
Timing is another issue. Some claim humans reached the Americas south of the ice sheets well before the usually accepted 16-14 kya timeframe11 12, while others claim extinct megafauna survived on the mainland into the Holocene13. Both are used to challenge overkill or at least its rapid (blitzkrieg) version13 14. However, these claims remain controversial. I have addressed the early human arrival claims before and will do so again in part 2.
Studies often claim evidence of megafauna surviving on the American mainland after 10,000 years before present, but many are inconclusive or dubious. Often, they rely on sedimentary DNA detected in soil samples15, but dating these sediments is problematic due to reworking, which can yield erroneously young survival dates16. Late dates from directly testing fossils are also usually based on flawed methods and tend to be refuted upon retesting13.
While such findings are understandably exciting for researchers and science enthusiasts, it is best to exercise the same caution and scrutiny towards them as with anything else in science. Further, regardless of when humans first set foot in the Americas or when the last members of extinct species died off, we have enough evidence to say that an abrupt collapse of megafaunal populations took place during the glacial-interglacial transition17 18.
We will start by looking at the patterns surrounding this collapse, and see whether climate fits as the central cause.
Unprecedented Losses
At first glance, climate change seems like a compelling explanation for the extinctions in the Americas. These losses occurred during significant global climate shifts as the ice age ended. The massive North American ice sheet melted rapidly, pushing cold-adapted plants and animals northward.
While dramatic, the environmental transformations during this period were not unprecedented. Similar glacial-interglacial transitions had occurred over hundreds of thousands of years without causing megafaunal losses on this scale6 19. The survival of past animal communities through these cycles is an enduring objection to climate-based explanations.
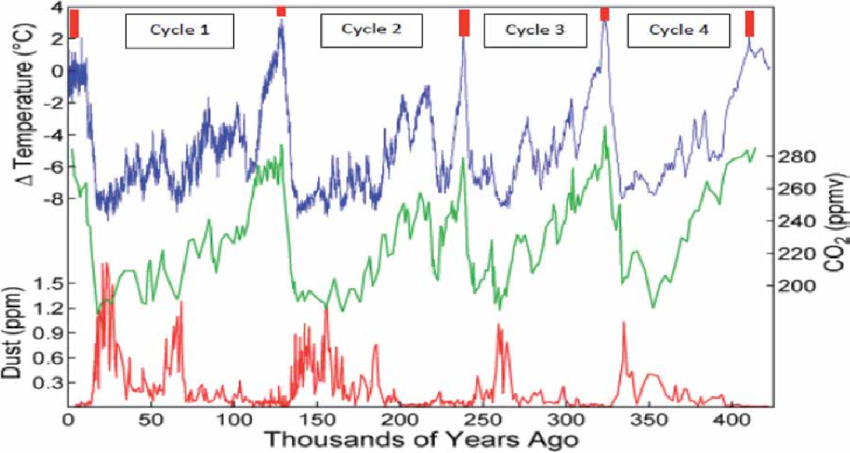
Moreover, the sheer number of extinctions does not fully capture how anomalous this event was-details about which groups disappeared are quite telling. Certain taxa had thrived in the New World for millions of years: proboscideans for 16 million, ground sloths for 35 million, glyptodonts for 38 million, litopterns for 62 million, and horses for 56 million. Yet, not a single species from these groups survived. Most vanished by the early Holocene, with a few lingering on islands until the mid-Holocene20 21 .
The abrupt loss of such long-established taxa in a geologic instant is so unprecedented in Quaternary history, and perhaps most of Earth’s history, that it requires a better explanation than standard climate cycles alone.
Uneven Climate Change
Comparisons across continents are just as important as comparisons across time. But continents have experienced varying durations of anatomically modern human (Homo sapiens) and hominin occupation, as well as distinct climates and climate-change patterns, so this must be done carefully to minimize confounding variables. Therefore, as stated in the introduction, this post will extensively compare and contrast North and South America.
Why? Both continents were settled by the same group of people, known as Paleo-Indians, around the same time, with no prior hominin presence18 22. However, the way each experienced climate change differed significantly due to different geographical realities. While North America often dominates discussions on extinction, South America provides crucial context for understanding the respective roles of human activity and climate change across both regions.
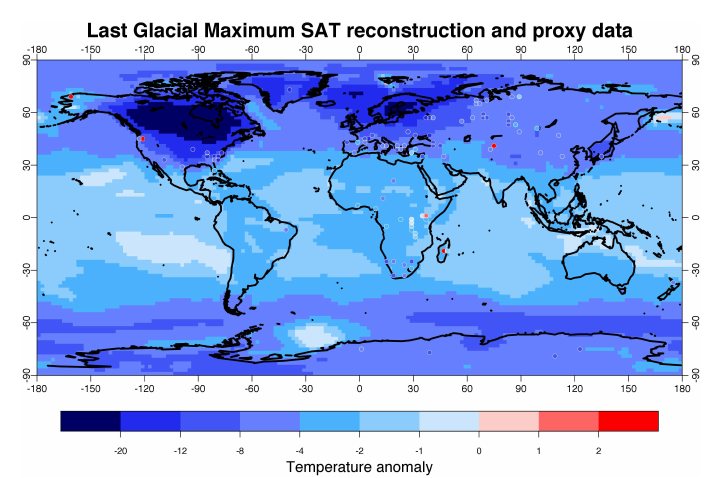
South America is less climatically turbulent than the mid-upper latitudes of North America23 24. It has a favorable geographic location with most of it being located in the relatively stable southern hemisphere and the part that extends into the northern hemisphere still falling well within the tropics. Glaciations were relatively minor in South America, being restricted to the Andes. In this regard at least, South America is more comparable to Africa or Australia than North America.
Temperature differences between the Last Glacial Maximum and present day were less pronounced in South America than North America25. Environmental changes during the transition to the Holocene largely reflect this pattern. In parts of North America, profound changes took place: areas that were once covered in glaciers were claimed by frigid tundra, then boreal forest, and eventually temperate forest26. In South America, biomes largely reflected modern day distributions a noticeable exception being that cool, moist adapted plants that were once widespread became rare outside of high-altitude regions27.
Warm-climate vegetation zones would have certainly expanded somewhat poleward in South America, but the main environmental shift was a back and forth shifting of grassy and forested biomes. However, despite a reduction in area compared to the LGM, grasslands and savannahs persisted through the transition and are still present today as vast ecosystems like the Cerrado, Llanos, Beni savannah, Pampas, and others.
All of these details are crucial because, despite South America’s relatively modest climate and environmental changes compared to North America, its megafauna experienced even more severe extinctions10. The implications of the mismatch between the magnitude of megafaunal loss and climate shifts in South America are often ignored by climate theorists.
With that in mind, let us zoom in to see the issues with climate scenarios for each continent.
Environmental Changes in North America
In North America, there were a myriad of species with wide distributions and varied diets who happened to go extinct. Their fates present a major challenge for climate theories, as one would expect environmental stress to be less harsh on generalists. Arctodus simus, or the giant short-faced bear, is a prime example, ranging from the Pacific to the Atlantic and from central Mexico to (periodically) northern Alaska. Naturally for such a widespread species, it would have survived on a plethora of different food sources and lived in various habitats from tundra to subtropical forest.
Other megafauna, like Megalonyx (Jefferson’s ground sloth), mastodons, and Camelops (a camel) had similar ranges, reaching as far south as Mexico and Central America and-during interglacials-as far north as Alaska and the Yukon. Naturally, their diets varied—what mastodons and Megalonyx in the Cypress swamps of Florida ate differed from what their counterparts in the Canadian taiga ate. Even animals that did not extend that far north, like Smilodon fatalis, dire wolves, and Columbian mammoths, were still highly adaptable and widespread.

This is only a small selection of flexible, broad-ranging species that went extinct, which raises a crucial question: What was so unique about the last glacial-interglacial transition that caused them to disappear completely? The Younger Dryas stadial, a sudden return to severe cold following the Bølling-Allerød warming, is often cited as a cause19. While this event may have impacted temperate and warm-adapted species, YD-like events involving a weakening or shutdown of the Atlantic Gulf Stream circulation were an intrinsic part of climate cycles for hundreds of thousands of years28 29, with the third to last glacial termination (Termination 3) showing a remarkably similar pattern to the last one.
Further, the Younger Dryas affected more than just North America—Europe saw even greater climate shifts due to its position downwind of the Atlantic30. Yet, temperate species there like red deer, wild boar, ibex, aurochs, and wisent survived the Younger Dryas. In fact, the European animals that did go extinct during the transition were primarily cold-adapted ones that relied on the disappearing mammoth steppe. This contrasts sharply with North America, where losses were amongst primarily temperate and warm-adapted species living in a variety of habitats.
Further, the distribution of vegetation, perhaps moreso than climate itself, shapes faunal distribution as animals rely on vegetation for food and habitat. The movement of vegetation zones can lag climatic shifts for millennia, and while deglaciation began around 17 kya, North America’s biome map did not fully resemble its modern form until ~8-7 kya26. This is very significant-the megafaunal collapse in North America occurred around 13-11 kya and was abrupt18.
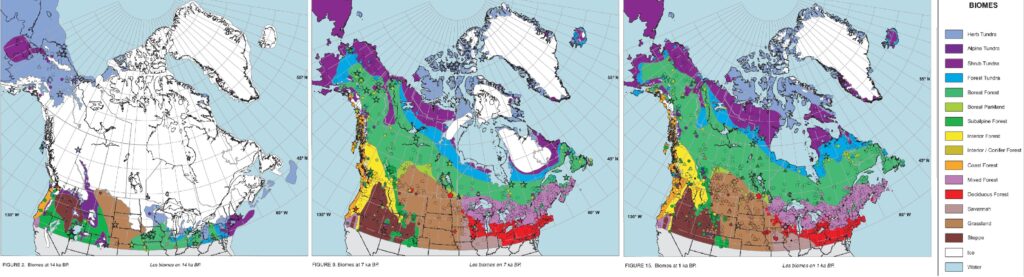
A lot of special attention has been given to eastern Beringia (Alaska and the Yukon), which was situated northwest of the ice sheets and included part of the mammoth steppe31 32. The grazers of this region (woolly mammoth, horse) appear to have declined when increased moisture associated with the Bølling–Allerød interstadial promoted shrubby tundra at the expense of steppe-tundra32. While I do not think it is likely that environmental changes alone doomed these animals (as they survived previous interglacials), it should be noted that Beringia is a complete outlier in terms of its environmental history.
Beringia is situated at high-latitudes, and there is no far northern equivalent of the vast stable grasslands that persist into interglacials as seen in mid-latitude North America like the Great Plains, so it is not surprising that grazers living there would struggle to cope with the expansion of shrub tundra and eventually forest. However, it should be understood that this dynamic is not applicable to all of North America. Therefore, over-extrapolating from Beringia and the Arctic in general is not advisable.
Environmental Changes in South America
As stated earlier, the mismatch between climate change magnitude and extinction rate in South America remains a serious challenge for climate theorists. However, some have attempted environmental explanations for extinctions in South America anyway, although they do include a role for humans to make them more compelling. According to the so-called “broken zig-zag” hypothesis, closed environments like rainforest expanded greatly in place of the savannah and grasslands that most megafauna relied on33. The increasingly small and fragmented patches of open areas then made them vulnerable to human hunters.

Addressing this argument is slightly complicated. In North America, this logic would not hold up as we know of iconic megafauna like mastodons and Megalonyx who inhabited closed forests34 35 but died off anyway. In South America, however, there is uncertainty about which megafaunal species inhabited tropical rainforests36. This is due to very poor preservation of fossils in such environments and ongoing debates about the Amazon’s extent during glacials.
However, there are important considerations. In nearby Costa Rica, the proboscidean Cuvieronius hyodon clearly dwelled in closed forests and fed on C3 vegetation37. Although it is unclear whether these were true rainforests or more seasonal forests, it should be noted that there is a diversity of forest types in Central and South America and extinct megafauna clearly lived in at least some of them. Second, large herbivores inhabit rainforests in Africa and Asia today, making it hard to imagine that exceptionally biodiverse South American rainforests38 did not host extinct megafauna.
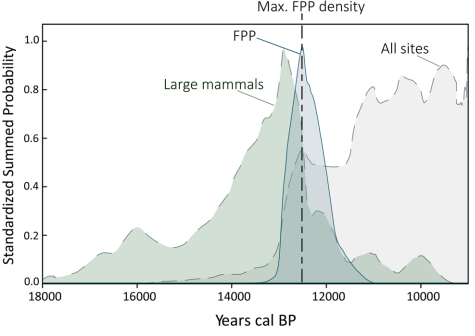
But what can we say about actual rainforest extent during the extinction period? According to Prates and Perez (2021), megafaunal populations crashed abruptly between 12.9 and 10.9 kya39, from the terminal Pleistocene to the very early Holocene. Pollen-based vegetation reconstructions give the best direct view of what the environment of a place looked at a given time and can give us a rough idea of how dominant rainforest was.
The details and nuances surrounding these vegetation changes are too many to fit into this piece, but one can read my guest article on The Extinctions blog where I show how rainforest extent in tropical South America did not expand nearly as much by the start of the extinctions as many seem to believe. As a matter of fact, newer climate-based simulations of past vegetation show that for even much of the Holocene, South America had less rainforest area than today40. This does not fit the picture of animals being squeezed in by an ever-expanding rainforest.
Moreover, in the Andes, Patagonia, and Pampas, the population crash followed millennia of rising megafaunal density after a low during peak glacial conditions39. One possibility is that this may be due to improving conditions for animals such as greater plant productivity, or this may reflect taphonomic bias as animals closer to the present are more likely to be preserved as fossils. However, the rise (see below) is steep enough that, at a minimum, we can rule out the notion that animal populations were meaningfully decreasing in these particular regions (and perhaps South America more broadly).
Another aspect to consider in this story involves the dry forests like the Gran Chaco and Caatinga. An intriguing possibility is that South America’s modern distribution of dry forests vs. savannahs may not be entirely natural. Studies indicate that plants in drier areas exhibit anti-herbivory traits41, suggesting they were once heavily consumed by megafauna. Another study finds that South America has far denser tree cover than Africa at comparable moisture levels, potentially because of the absence of large herbivores that would maintain open habitats in the former42.

Some dry forests in South America already contain numerous patches of savannah41. The authors of the second study suggest that if South America’s megafauna still existed, the dry forests could possibly be more similar to savannah broadly, while presently-defined savannahs might be less woody42. This would mean a much-less woody continent overall.
There are therefore three aspects which undermine the notion that rainforest expansion played a major part in the extinction of South America megafauna. First, extinctions seem to have occurred across a wide variety of ecosystems-not just savannahs-and there is no good reason to assume that rainforests were not significantly affected as well, as an absence of megafaunal fossils does not mean that extinct megafauna were not present there. Second, rainforest extent in South America at the time of extinctions was not unusually great. Third, animals do have an ability to shape their environments, and we can reasonably speculate that wooded regions assumed to be unsuitable for open-habitat megafauna today may have been more open, and hence more suitable, if those megafauna had simply not gone extinct.
Therefore, we can say that a viable climatic explanation for extinction in South America is lacking.
The Big Picture: Extirpation vs. Extinction
After having discussed the changes in individual continents, we can once again take a broader view to consider overarching trends. A very important aspect to keep in mind regarding this topic is the difference between extirpation and extinction. Extirpation refers to regional disappearance of a taxon, whereas extinction proper refers to its complete elimination. The issue when trying to link local faunal shifts to broader patterns is how complex environmental changes can be. A species’ preferred habitat may shrink in one region but remain stable or even expand elsewhere, which can partially or fully offset declines in the former.
While some animals undoubtedly had a hard time during the glacial-interglacial transition as a result of habitat reduction-like the grazers of Beringia-the evidence that this was happening to most species across the board in the Americas is severely lacking. The transition period provided ample habitat for grazers and browsers south of the ice sheets26, although detrimental changes post-extinction of large animals cannot be excluded6.
Due to the rich assemblage of megafaunal fossils yielded from the La Brea tar pits in Los Angeles, southern California has become a prime focus of research on the Late Pleistocene extinctions. One study describes climate (along with human agency) as having been instrumental in extinctions here due to hotter and drier conditions associated with the deglaciation phase5. However, while it may be true that climate change resulted in range shifts and extirpations there, drawing conclusions from this about extinction at the continental level would be problematic. The very same animals that were present in southern California-dire wolves, American lions, Smilodon fatalis, and mastodons-should have benefitted from habitat expansion in other parts of their North American ranges, such as the areas freed from glacial ice. Yet, they died off there as well.
Patagonia offers a good case study for why localized changes should not be assumed to reflect broader trends. A paper postulated that the transition of a section of southern Chile from cold grassland to Nothofagus forest between 13-11 kya was partly responsible for the extinction of grazers there like Mylodon, Lama gracilis, and Hippidion saldiasi 43. However, a larger nearby region in Argentina retained cold steppes through and beyond this period44, suggesting that the transition from grassland to forest east of the Andes took place along a fairly narrow strip of land. In other words, there was no shortage of grassland in Patagonia at any time.

And while sea level rise flooded part of eastern Patagonia, megafaunal density appears to have been on the rise from the end of LGM conditions through the late glacial39. The dramatic increase in density took place from 18 kya until around 13 kya when it crashed in a sudden and unnatural fashion. Thus, losses in land were potentially being offset by improved living conditions for megafauna, such as higher vegetative productivity. When combining the persistence of vast steppes through the late glacial and beyond with the likely scenario of vegetative productivity improving, it is difficult to draw a clear link between habitat shifts and extinction in Patagonia.
All of this demonstrates the give and take nature of habitat shifts in the face of climate change. An informed perspective looking at overarching animal-habitat dynamics rather than localized ones explains why continent-scale correlations between climate and extinction in the Americas are weak or nonexistent22.
Size-Biased Extinctions
It is no secret that the Late Pleistocene extinctions as a whole were heavily size-biased18 33 45. The Americas were no exception in this regard. In fact, there are salient points that drive home exactly how absurdly size-biased the extinctions were in the New World, which raise further doubts about the role of climate as we would expect there to be a somewhat analogous loss of small animals in the event of primarily climate-driven losses like in previous natural extinctions.
A striking fact is that not a single species of megamammal (animal weighing over 1000 kg) survived in the Americas, from Patagonia to the Arctic6 33. American megamammals belonged to diverse groupings, such as proboscideans, giant ground sloths, and glyptodonts. This weight class included species with a wide range of habitats and diets6, making it hard to see how they should have been so susceptible to a typical glacial-interglacial transition.
South America, the most biodiverse continent46, highlights the size paradox in a surprising but rarely mentioned way. While South American rainforests like the Amazon are renowned for their unparalleled species richness, the tropical/subtropical grassy biomes of South America are also quite biodiverse. Despite losing nearly all of their megafauna, they almost rival the mammalian diversity of eastern and southern African savannahs38 42, which retained most of their megafauna. This is because South America, conveniently, managed to preserve its small mammal diversity.
Therefore, climate theorists must address two glaring questions: 1) Why did climate change not spare a single megamammal in the New World and 2) Why did climate change allow South America to retain unequaled small mammal diversity while wiping out nearly all of its large mammals? The size-biased nature of the extinction event remains another problem for environmental theories.
Fecundity
In North America, surviving herbivore species often have shorter gestation periods-which allow for faster reproduction-than their extinct counterparts did. While gestation length generally correlates with size, this pattern holds even when controlling for it. For instance, horses have gestation periods of 11–12 months and camels around 13 months, and this was probably true for their extinct American relatives. Compared to bison, American horses were smaller and Camelops was a similar weight. Yet, bison persisted and the other two did not. Bison, as well as other survivors like elk, moose, caribou, and pronghorn, have gestation periods of under 300 days.
A study speculated that bison survived slightly longer than horses in eastern Beringia due to their “mixed-feeding” habits, but isotopic and dental wear analyses of Beringian horses and bison show that both animals had very similar diets47 48. Further, both bison and horse were present on the grasslands of the Great Plains further south but only the former survived, casting doubt on dietary explanations and suggesting a stronger link with reproductive rates.


The survival of species with shorter gestation periods does not align with climatic explanations, as it is hard to see why resource-limited environments would favor more fecund species over slower-reproducing ones.
American Resilience?
With all the talk of environmental change affecting animals in the past, it may be surprising to hear that the Americas were actually blessed in some ways as far as allowing animals to cope with these shifts. But this is exactly what I would argue on the basis of the favorable geographical arrangements on both continents, even in North America in spite of its extreme glacial advances and retreats.
Today, there is a vast expanse of flat grassland in central North America stretching from the Texas Gulf coast all the way to Alberta in Canada-the aforementioned Great Plains. The grassland grades into forest at its eastern boundary, and the forest continues eastwards all the way to the Atlantic. The climate in both the forest and grassland regions of North America ranges from fairly warm in the south to quite cold in the north.
The grassland and forest belts existed during all periods of the Late Pleistocene as well. Although their exact distributions shifted, the grassland was centrally located and the forest further east. Very importantly, there is no geographic barrier separating the two belts. Animals could move west or east as they needed to track changes in vegetative openness. Due to the north-south arrangement of these belts and a lack of east-west mountain ranges, temperate North American animals could track changes in temperature as well.
In South America, the spatial arrangement of biomes is different but there is a similarity in that within the tropical/subtropical zone, the once megafauna-dense savannah, grassland, and scrub are not separated from the major rainforests by geographical barriers. Prominent mountain ranges or east of the Andes are rare, and true deserts are only along the Pacific coast and in parts of Patagonia. This allows animals to easily move to more suitable areas when the climate changes.
In both cases, there is a stark contrast to Eurasia where the peculiar arrangement of water bodies, mountains, and vegetation zones would create significant obstacles for plants and animals coping with periods of environmental duress. For example, despite both regions lying in the temperate zone, Europe features a stronger latitudinal gradient in terms plant genetic diversity compared to eastern North America as it was more strongly affected by millennial-scale climate variability, as well as the impact of east-west mountain ranges on dispersal49 50. The role of Eurasia’s climatic and geographic complexity in its own extinction patterns is a topic worthy of discussion at another time, but the point here is that neither North or South America were places where coping with climate change was unusually difficult for the plants and animals.
We see more evidence for this when we look at the size of megafauna present in the western hemisphere. Gary Haynes has described how severe climatic stress should cause survivors to shrink due to declining food sources19. Yet, we see that in the Late Pleistocene, the mean mass of animals in both North and South America was similar to that of Africa45. This is after enduring multiple similar glacial-interglacial cycles and reinforces the idea that they weathered such changes effectively.

The Case for a Predominantly Human Causation
Let us restate the peculiar features of the extinction event:
- It is the only glacial-interglacial transition to feature such high losses of megafauna.
- Diverse and extremely long-lasting taxa disappeared in a geologic instant.
- The severity of extinctions did not correlate with the magnitude of climatic changes.
- The mechanisms through which climate-driven habitat changes would have led to extinction are either unknown or unconvincing.
- Extinctions were extremely size-biased.
- Survivors tended to have lower gestation periods even controlling for size.
All of these facts are hard to reconcile with a climate-driven extinction but can be explained effortlessly with a human-driven one. This transition was uniquely deadly due to the impact of humans. Extinct species, including generalists and long-lasting taxa, were all highly vulnerable as they faced a novel threat. All regions suffered losses regardless of environmental conditions because they were all settled by people. Size-biased extinctions are expected, as larger animals are more susceptible to human predation45 51. The chance of survival is positively correlated with a shorter gestation period, because these animals offset losses from hunting more easily.
Attempting to explain the extinctions via climate instead of humans invariably results in glaring holes. In one study, researchers analyzed extinction patterns across continents and determined that there was a link between climate footprint size and extinction intensity24. The big outlier was South America, which despite having a small climatic footprint, had a high extinction rate (Australia would have been another if it were included).
As both North and South America were settled by the same group of people at around the same time period, it would be quite strange if climate played a much larger role in the extinction of North America’s megafauna compared to South America’s, especially since extinctions were in fact slightly less severe in North America.

For example, while the Younger Dryas cold snap is often blamed for North American extinctions, South America south of the equator was actually experiencing gradual warming at the time19 39. Yet extinctions still occurred there near-simultaneously. Therefore, the coincidence of North American extinctions with the Younger Dryas should not automatically be taken as proof that it, or any climate event, was the responsible force.
Non-climate factors can even explain South America’s greater extinction rate. South American megafauna were larger on average than North American ones, and we know the extinctions were size-biased. Additionally, native (pre-Great American Biotic Interchange) South American animals evolved in a milieu with far less dangerous predators than their North American counterparts52, potentially making them more defenseless against the human super-predator. The fact that almost all surviving mammalian megafauna in South America-deer, tapirs, big cats, llama relatives, etc.- are North American migrants from the GABI33 would further support this point.
A False Middle Ground?
Throughout this post, I have referenced the common view that both climate and humans played substantial roles in American megafauna extinctions3 4 33. By avoiding single-cause explanations, these perspectives appear nuanced and intuitive, and often come in the form of describing these factors as “synergistic” rather than additive33. Even many overkill proponents posit a climatic influence53.
Although I admire and cite many experts who hold this view, I take issue with the reasoning behind it. First, Australia demonstrates that a major input from climate change is not required for continental extinctions. Second, we must clarify what it means for a factor to be “necessary” or to have “contributed.” If it simply means climate and humans influenced the timing and specifics of extinctions, then it is understandable. But if it implies that most megafauna could have survived into the modern era if dealing with only one factor rather than both, it is questionable.
If climate had a large but not exclusive input in driving extinction (say 30–50%), we should still see a noteworthy correlation between climate change intensity and extinction severity, or between species specialization and vulnerability. Yet, such patterns are almost entirely absent. While some animals may have been highly sensitive to climate shifts, their dynamics are certainly not representative of all species in the Americas.
The overall evidence suggests that human-related factors (which I will expand on in the next post) sufficiently explain broad extinction patterns. At present, I would argue climate change’s overall impact was likely minimal. Multifactor hypotheses remain popular, partly because they are harder to refute than climate-centric models which have become increasingly untenable. Regardless of whether one favors a major or minor role for climate, an objective assessment would recognize that human actions doomed at least a large portion of the New World megafauna independent of environmental conditions.
For example, we can debate what climate changes put pressure on woolly mammoths in the Yukon or Pacific mastodons in California, but at the end of the day, no proboscidean survived anywhere in the New World. The same applies to ground sloths, horses, glyptodonts, and others. The fact that entire orders and families were eliminated in such a short span of time means that environmental factors affecting individual species within these groups were essentially irrelevant at the higher level.
Study after study has been published debating the relative impact of climate on the extinction of individual species or animal communities, often focusing on specific regions of North or South America. However, the fact that more or less the same outcome (high rate of extinction) occurred everywhere regardless of different climate characteristics and that the extinctions wiped out whole families (despite high dietary and habitat variation within said families) ultimately means that climate could not have been more than a weak secondary contributor.
Conclusion and Sequel
In this post, we have established the weakness of climate change in comparison to humans in explaining the Late Pleistocene extinction mystery in the Americas. Despite the indisputable presence of major climate shifts during the extinction window, the evidence overwhelmingly rules out climate as the dominant factor in megafaunal loss. Multifactor theories wherein climate was a significant component alongside humans are popular but ultimately suffer from many of the same issues.
Big-picture thinking is key to solving the debate. As I have shown here, continent-wide analysis is necessary, and appropriate comparisons between continents can be valuable-as with North and South America. North America’s losses cannot be analyzed without also considering South America’s extinction patterns, which throw a wrench into climate narratives.
However, there is still much more to explore on this topic. The evidence for human predation on megafauna, the intricate relationship between human settlement and megafaunal decline, and the specific mechanisms driving these extinctions all warrant further discussion. These aspects will be the focus of the next installment in this series.
References
1. Martin, P. S. (1973). The Discovery of America: The first Americans may have swept the Western Hemisphere and decimated its fauna within 1000 years. Science, 179(4077), 969–974. https://doi.org/10.1126/science.179.4077.969
2. Grayson, D. K., & Meltzer, D. J. (2003). A requiem for North American overkill. Journal of Archaeological Science, 30(5), 585–593. https://doi.org/10.1016/s0305-4403(02)00205-4
3. Metcalf, J. L., Turney, C., Barnett, R., Martin, F., Bray, S. C., Vilstrup, J. T., Orlando, L., Salas-Gismondi, R., Loponte, D., Medina, M., De Nigris, M., Civalero, T., Fernández, P. M., Gasco, A., Duran, V., Seymour, K. L., Otaola, C., Gil, A., Paunero, R., & Prevosti, F. J. (2016). Synergistic roles of climate warming and human occupation in Patagonian megafaunal extinctions during the Last Deglaciation. Science Advances, 2(6), e1501682. https://doi.org/10.1126/sciadv.1501682
4. Broughton, J. M., & Weitzel, E. M. (2018). Population reconstructions for humans and megafauna suggest mixed causes for North American Pleistocene extinctions. Nature Communications, 9(1). https://doi.org/10.1038/s41467-018-07897-1
5. F. Robin O’Keefe, Dunn, R. E., Weitzel, E. M., Waters, M. R., Martinez, L., Binder, W. J., Southon, J., Cohen, J. E., Meachen, J., Larisa R.G. DeSantis, Kirby, M. E., Ghezzo, E., Brenner-Coltrain, J., Fuller, B. T., Farrell, A. B., Takeuchi, G. T., MacDonald, G. M., Davis, E. B., & Lindsey, E. (2023). Pre–Younger Dryas megafaunal extirpation at Rancho La Brea linked to fire-driven state shift. Science, 381(6659). https://doi.org/10.1126/science.abo3594
6. Owen-Smith, N. (1987). Pleistocene extinctions: the pivotal role of megaherbivores. Paleobiology, 13(3), 351–362. https://doi.org/10.1017/s0094837300008927
7. Pires, M. M., Koch, P. L., Fariña, R. A., de Aguiar, M. A. M., dos Reis, S. F., & Guimarães, P. R. (2015). Pleistocene megafaunal interaction networks became more vulnerable after human arrival. Proceedings of the Royal Society B: Biological Sciences, 282(1814), 20151367. https://doi.org/10.1098/rspb.2015.1367
8. Holliday, V. T., Daulton, T. L., Bartlein, P. J., Boslough, M. B., Breslawski, R. P., Fisher, A., Jorgeson, I., Scott, A. C., Koeberl, C., Marlon, J. R., Severinghaus, J. P., Petaev, M. I., & Claeys, P. (2023). Comprehensive refutation of the Younger Dryas Impact Hypothesis (YDIH). Earth-Science Reviews, 104502–104502. https://doi.org/10.1016/j.earscirev.2023.104502
9. Kathleen Lyons, S., Smith, F. A., Wagner, P. J., White, E. P., & Brown, J. H. (2004). Was a “hyperdisease” responsible for the late Pleistocene megafaunal extinction?. Ecology Letters, 7(9), 859–868. https://doi.org/10.1111/j.1461-0248.2004.00643.x
10. Koch, P. L., & Barnosky, A. D. (2006). Late Quaternary Extinctions: State of the Debate. Annual Review of Ecology, Evolution, and Systematics, 37(1), 215–250. https://doi.org/10.1146/annurev.ecolsys.34.011802.132415
11. Bennett, M. R., Bustos, D., Pigati, J. S., Springer, K. B., Urban, T. M., Holliday, V. T., Reynolds, S. C., Budka, M., Honke, J. S., Hudson, A. M., Fenerty, B., Connelly, C., Martinez, P. J., Santucci, V. L., & Odess, D. (2021). Evidence of humans in North America during the Last Glacial Maximum. Science, 373(6562), 1528–1531. https://doi.org/10.1126/science.abg7586
12. Thaís Rabito Pansani, Pobiner, B., Guériau, P., Mathieu Thoury, Tafforeau, P., Baranger, E., Águeda Vilhena Vialou, Vialou, D., McSparron, C., Castro, M. C., Trindade, A., Bertrand, L., & Alves, L. (2023). Evidence of artefacts made of giant sloth bones in central Brazil around the last glacial maximum. Proceedings of the Royal Society B: Biological Sciences, 290(2002). https://doi.org/10.1098/rspb.2023.0316
13. Politis, G. G., Messineo, P. G., Stafford, T. W., & Lindsey, E. L. (2019). Campo Laborde: A Late Pleistocene giant ground sloth kill and butchering site in the Pampas. Science Advances, 5(3), eaau4546. https://doi.org/10.1126/sciadv.aau4546
14. Bampi, H., Barberi, M., & Lima-Ribeiro, M. S. (2022). Megafauna kill sites in South America: A critical review. Quaternary Science Reviews, 298, 107851. https://doi.org/10.1016/j.quascirev.2022.107851
15. Haile, J., Froese, D. G., MacPhee, R. D. E., Roberts, R. G., Arnold, L. J., Reyes, A. V., Rasmussen, M., Nielsen, R., Brook, B. W., Robinson, S., Demuro, M., Gilbert, M. T. P., Munch, K., Austin, J. J., Cooper, A., Barnes, I., Möller, P., & Willerslev, E. (2009). Ancient DNA reveals late survival of mammoth and horse in interior Alaska. Proceedings of the National Academy of Sciences, 106(52), 22352–22357. https://doi.org/10.1073/pnas.0912510106
16. Rawlence, N. J., Lowe, D. J., Wood, J. R., Young, J. M., Churchman, G. J., Huang, Y.-T., & Cooper, A. (2014). Using palaeoenvironmental DNA to reconstruct past environments: progress and prospects. Journal of Quaternary Science, 29(7), 610–626. https://doi.org/10.1002/jqs.2740
17 Surovell, T. A., Pelton, S. R., Anderson-Sprecher, R., & Myers, A. D. (2015). Test of Martin’s overkill hypothesis using radiocarbon dates on extinct megafauna. Proceedings of the National Academy of Sciences, 113(4), 886–891. https://doi.org/10.1073/pnas.1504020112
18. Prates, L., & Perez, S. I. (2024). The Evidence for Human Agency in the Late Pleistocene Megafaunal Extinctions. Encyclopedia of the Anthropocene, 219–226. https://doi.org/10.1016/B978-0-443-14082-2.00035-1
19. Fiedel, S., & Haynes, G. (2004). A premature burial: comments on Grayson and Meltzer’s “Requiem for overkill.” Journal of Archaeological Science, 31(1), 121–131. https://doi.org/10.1016/j.jas.2003.06.004
20. Graham, R. W., Belmecheri, S., Choy, K., Culleton, B. J., Davies, L. J., Froese, D., Heintzman, P. D., Hritz, C., Kapp, J. D., Newsom, L. A., Rawcliffe, R., Saulnier-Talbot, É., Shapiro, B., Wang, Y., Williams, J. W., & Wooller, M. J. (2016). Timing and causes of mid-Holocene mammoth extinction on St. Paul Island, Alaska. Proceedings of the National Academy of Sciences, 113(33), 9310–9314. https://doi.org/10.1073/pnas.1604903113
21. Steadman, D. W., Martin, P. S., MacPhee, R. D. E., Jull, A. J. T., McDonald, H. G., Woods, C. A., Iturralde-Vinent, M., & Hodgins, G. W. L. (2005). Asynchronous extinction of late Quaternary sloths on continents and islands. Proceedings of the National Academy of Sciences, 102(33), 11763–11768. https://doi.org/10.1073/pnas.0502777102
22. Araujo, B. B. A., Oliveira-Santos, L. G. R., Lima-Ribeiro, M. S., Diniz-Filho, J. A. F., & Fernandez, F. A. S. (2017). Bigger kill than chill: The uneven roles of humans and climate on late Quaternary megafaunal extinctions. Quaternary International, 431, 216–222. https://doi.org/10.1016/j.quaint.2015.10.045
23. Herrando-Moraira, S., Nualart, N., Galbany-Casals, M., Garcia-Jacas, N., Ohashi, H., Matsui, T., Susanna, A., Tang, C. Q., & López-Pujol, J. (2022). Climate Stability Index maps, a global high resolution cartography of climate stability from Pliocene to 2100. Scientific Data, 9(1), 48. https://doi.org/10.1038/s41597-022-01144-5
24. Nogués-Bravo, D., Ohlemüller, R., Batra, P., & Araújo, M. B. (2010). Climate Predictors of Late Quaternary Extinctions. Evolution, no-no. https://doi.org/10.1111/j.1558-5646.2010.01009.x
25. Annan, J. D., & Hargreaves, J. C. (2013). A new global reconstruction of temperature changes at the Last Glacial Maximum. Climate of the Past, 9(1), 367–376. https://doi.org/10.5194/cp-9-367-2013
26. Dyke, A. S. (2007). Late Quaternary Vegetation History of Northern North America Based on Pollen, Macrofossil, and Faunal Remains. Paleoenvironments, 59(2-3), 211–262. https://doi.org/10.7202/014755ar
27. Luiz, J., Nigel, Cruz, F. W., Akabane, T. K., Maria, Augusto José Pereira-Filho, Grohman, C. H., Reis, L. S., Ferreira, E. S., Gregório C. T. Ceccantini, & De, E. (2024). Humid and cold forest connections in South America between the eastern Andes and the southern Atlantic coast during the LGM. Scientific Reports, 14(1). https://doi.org/10.1038/s41598-024-51763-8
28. Sima, A., Paul, A., & Schulz, M. (2004). The Younger Dryas—an intrinsic feature of late Pleistocene climate change at millennial timescales. Earth and Planetary Science Letters, 222(3-4), 741–750. https://doi.org/10.1016/j.epsl.2004.03.026
29. Broecker, W. S., Denton, G. H., Edwards, R. L., Cheng, H., Alley, R. B., & Putnam, A. E. (2010). Putting the Younger Dryas cold event into context. Quaternary Science Reviews, 29(9-10), 1078–1081. https://doi.org/10.1016/j.quascirev.2010.02.019
30. Renssen, H., Goosse, H., Roche, D. M., & Seppä, H. (2018). The global hydroclimate response during the Younger Dryas event. Quaternary Science Reviews, 193, 84–97. https://doi.org/10.1016/j.quascirev.2018.05.033
31. Cooper, A., Turney, C., Hughen, K. A., Brook, B. W., McDonald, H. G., & Bradshaw, C. J. A. (2015). Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science, 349(6248), 602–606. https://doi.org/10.1126/science.aac4315
32. Monteath, A. J., Gaglioti, B. V., Edwards, M. E., & Froese, D. (2021). Late Pleistocene shrub expansion preceded megafauna turnover and extinctions in eastern Beringia. Proceedings of the National Academy of Sciences, 118(52). https://doi.org/10.1073/pnas.2107977118
33. Cione, A. L., Tonni, E. P., & Soibelzon, L. (2009). Did humans cause the Late Pleistocene-Early Holocene mammalian extinctions in South America in a context of shrinking open areas? American megafaunal extinctions at the end of the Pleistocene, 125-144.
34. Karpinski, E., Hackenberger, D., Zazula, G., Widga, C., Duggan, A. T., Golding, G. B., Kuch, M., Klunk, J., Jass, C. N., Groves, P., Druckenmiller, P., Schubert, B. W., Arroyo-Cabrales, J., Simpson, W. F., Hoganson, J. W., Fisher, D. C., Ho, S. Y. W., MacPhee, R. D. E., & Poinar, H. N. (2020). American mastodon mitochondrial genomes suggest multiple dispersal events in response to Pleistocene climate oscillations. Nature Communications, 11(1), 4048. https://doi.org/10.1038/s41467-020-17893-z
35. Semken, H. A., McDonald, H. G., Graham, R. W., Adrain, T., Artz, J. A., Baker, R. G., Bryk, A. B., Brenzel, D. J., Bettis, E. A., Clack, A. A., Grimm, B. L., Haj, A., Horgen, S. E., Mahoney, M. C., Ray, H. A., & Theler, J. L. (2022). Paleobiology of Jefferson’s Ground Sloth (Megalonyx jeffersonii) derived from three contemporaneous, ontogenetically distinct individuals recovered from Southwestern Iowa, U.S.A.. Journal of Vertebrate Paleontology, 42(1). https://doi.org/10.1080/02724634.2022.2124115
36. Doughty, C. E., Wolf, A., Morueta-Holme, N., Jørgensen, P. M., Sandel, B., Violle, C., Boyle, B., Kraft, N. J. B., Peet, R. K., Enquist, B. J., Svenning, J.-C., Blake, S., & Galetti, M. (2015). Megafauna extinction, tree species range reduction, and carbon storage in Amazonian forests. Ecography, 39(2), 194–203. https://doi.org/10.1111/ecog.01587
37. Víctor Adrián Pérez-Crespo, Laurito, C. A., Joaquín Arroyo-Cabrales, Valerio, A. L., Cienfuegos-Alvarado, E., & Otero, F. J. (2023). Feeding habits of the Gomphothere Cuvieronius hyodon in Costa Rica:A biochemical approach. Historical Biology, 36(4), 734–741. https://doi.org/10.1080/08912963.2023.2183856
38. Jenkins, C. N., Pimm, S. L., & Joppa, L. N. (2013). Global patterns of terrestrial vertebrate diversity and conservation. Proceedings of the National Academy of Sciences, 110(28), E2602–E2610. https://doi.org/10.1073/pnas.1302251110
39. Prates, L., & Perez, S. I. (2021). Late Pleistocene South American megafaunal extinctions associated with rise of Fishtail points and human population. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-22506-4
40. Jelena Maksic, Shimizu, M. H., de, M., Igor Martins Venancio, Cardoso, M., & Ferreira, F. S. (2018). Simulation of the Holocene climate over South America and impacts on the vegetation. 29(2), 287–299. https://doi.org/10.1177/0959683618810406
41. Dantas, V. L., & Pausas, J. G. (2022). The legacy of the extinct Neotropical megafauna on plants and biomes. Nature Communications, 13(1), 129. https://doi.org/10.1038/s41467-021-27749-9
42. Doughty, C. E., Faurby, S., & Svenning, J.-C. (2015). The impact of the megafauna extinctions on savanna woody cover in South America. Ecography, 39(2), 213–222. https://doi.org/10.1111/ecog.01593
43. Villavicencio, N. A., Lindsey, E. L., Martin, F. M., Borrero, L. A., Moreno, P. I., Marshall, C. R., & Barnosky, A. D. (2015). Combination of humans, climate, and vegetation change triggered Late Quaternary megafauna extinction in the Última Esperanza region, southern Patagonia, Chile. Ecography, 39(2), 125–140. https://doi.org/10.1111/ecog.01606
44. Brook, G. A., M. Virginia Mancini, Franco, N. V., Bamonte, F., & Ambrústolo, P. (2013). An examination of possible relationships between paleoenvironmental conditions during the Pleistocene–Holocene transition and human occupation of southern Patagonia (Argentina) east of the Andes, between 46° and 52° S. Quaternary International, 305, 104–118. https://doi.org/10.1016/j.quaint.2012.11.005
45. Lyons, S. K., Smith, F. A., & Brown, J. H. (2004). Of mice, mastodons and men: human-mediated extinctions on four continents. Evolutionary Ecology Research, 6(3), 339-358.
46. Ceballos, G. (2005). Global Mammal Conservation: What Must We Manage? Science, 309(5734), 603–607. https://doi.org/10.1126/science.1114015
47. Fox-Dobbs, K., Leonard, J. A., & Koch, P. L. (2008). Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records. Palaeogeography, Palaeoclimatology, Palaeoecology, 261(1-2), 30–46. https://doi.org/10.1016/j.palaeo.2007.12.011
48. Kelly, A., Miller, J. H., Wooller, M. J., Seaton, C. T., Druckenmiller, P., & DeSantis, L. (2021). Dietary paleoecology of bison and horses on the mammoth steppe of eastern Beringia based on dental microwear and mesowear analyses. Palaeogeography, Palaeoclimatology, Palaeoecology, 572, 110394. https://doi.org/10.1016/j.palaeo.2021.110394
49. Fastovich, David, et al. “Legacies of Millennial-Scale Climate Oscillations in Contemporary Biodiversity in Eastern North America.” Philosophical Transactions of the Royal Society B Biological Sciences, vol. 379, no. 1902, 7 Apr. 2024, https://doi.org/10.1098/rstb.2023.0012
50. Lumibao, Candice Y., et al. “Ice Ages Leave Genetic Diversity “Hotspots” in Europe but Not in Eastern North America.” Ecology Letters, vol. 20, no. 11, 24 Sept. 2017, pp. 1459–1468, https://doi.org/10.1111/ele.12853
51. Ben-Dor, M., & Barkai, R. (2024). A matter of fat: Hunting preferences affected Pleistocene megafaunal extinctions and human evolution. Quaternary Science Reviews, 331, 108660. https://doi.org/10.1016/j.quascirev.2024.108660
52. Faurby, S., & Svenning, J.-C. (2016). The asymmetry in the Great American Biotic Interchange in mammals is consistent with differential susceptibility to mammalian predation. Global Ecology and Biogeography, 25(12), 1443–1453. https://doi.org/10.1111/geb.12504
53. Haynes, G. (2013). Extinctions in North America’s Late Glacial landscapes. Quaternary International, 285, 89–98. https://doi.org/10.1016/j.quaint.2010.07.026
One thing I wanted to ask, which I’ve been struggling to answer recently, is how horses survived in Eurasia if their long gestation periods made them more vulnerable to human hunting. Presumably, they would’ve had a similar gestation period to today’s horses, considering at least some of them were literally the ancestors of today’s domestic horses. And as far as I know, domestication of the horse didn’t begin until several thousands of years after the end of the Pleistocene. How did Eurasian horses hold out against the human onslaught where their American counterparts succumbed?
You’ve made a wonderful series of blog posts on the Quaternary extinctions. I’ve been a proponent of human-dominant causes for them for a few years now, and reading your posts has only bolstered my advocacy for them. I look forward to reading more from you in the future!
Thank you Dan, I really appreciate this comment.
To answer the question, it probably has something to do with prolonged coexistence with hominins in Eurasia. However, going beyond that is quite tricky as we have very little knowledge of which precise (and most likely subtle) differences in adaptations could have been key and can only really speculate. For example, extended coexistence with hominins could have produced:
-Differences in build which allowed Eurasian (and African) equids to better escape human predation, and hence have a lower mortality rate.
-Better senses that would have allowed them to evade human hunters, such as a possible innate ability to recognize the scent or sound of humans.
-Potentially slightly shorter gestation periods and earlier age of sexual maturity in Old World horses even compared to New World counterparts.
And I want to stress, these potential differences could have been VERY subtle, but that’s enough to be decisive. The question of why any proboscideans survived in Africa and Asia while none survived in the Americas would probably have a very similar answer, although obviously the horse family was obviously not decimated quite as badly as the proboscideans were.
Hope that answers your question.