In the previous sections of this three-part series, we explored why humans, not climate, played the much larger part in the extinction of Late Quaternary megafauna. However, this rather obvious reality has been obscured by a swathe of scientific papers with questionable conclusions. In this final piece of my series, I will aim to foster skepticism towards research that prioritizes climate among extinction causes by showcasing the flaws in a selection of papers, as well as to explore how ideological biases may be playing a role in the debate as a whole.
Very often the issue with such papers that put climate at the forefront of extinctions is not in how the data itself was collected but rather that the researchers’ assumptions and conclusions are not justified by either their own data or the broader body of information on paleoclimatology and paleoecology, and that a lot of crucial information is commonly left out. Science is not perfect and while all or most studies may contain at least a few errors, the problems with these particular cases are so substantial that they undermine the authors’ arguments entirely.
The effect of this poor science is a massive distortion of the debate regarding the Late Quaternary Extinctions, such that many continue to underestimate the role of humans in the event. The hope here is to demonstrate that the popularity and prominence of climate-based explanations in the context of the extinction debate should not automatically be taken as proof of their legitimacy; as an extension, appeals to moderation are unwise.
With that said, we can move on to the first case study.
Case Study 1: Failed Climatic Predictors of Extinction
The first study we will critique today, by Nogués-Bravo and colleagues, was previously referenced in my post on American extinctions and has been cited 95 times at the time of this article1. The authors argue that the strength of climate change predicts the intensity of megafaunal extinctions by continent. They rank four continents—North America, South America, Africa, and Eurasia—in terms of overall genera lost as well as by large and small mammal genera lost, and then rank them by “climate footprint” which is another term for the magnitude of climate change they experienced. They find an overall trend of climate change footprint correlating with higher extinctions.
However, there is a massive problem: South America appears as a major outlier as it had a very high extinction rate but a low climatic footprint. The researchers at one point try to reconcile South America’s high extinction rate with its low climatic impact by arguing that perhaps climate may have been important in South America, because the far southern tip of the continent had a large climatic footprint and that most extinctions occurred in the southern part of the continent:
Our predictions do not match the extremely high levels of recorded extinctions
in South America, suggesting that nonclimatic factors are more
likely to have driven extinctions there. However, it should be noted
that in South America most of the known extinctions occurred in
the southern part of the continent. The average footprint value of
the land below 40◦S is 0.48, a value that our model also predicts
in areas of continents such as North America that suffered high
levels of extinctions (Fig. 1). The average climate footprint value
for the South American lands below 40◦S is indeed—three to four
times higher than the average climate footprint for South America
as a whole.
Aside from the strange decision to use the euphemism “non-climatic factors” to describe human impact, this explanation makes little sense; far southern Patagonia has very different climatic forcings compared to say the Pampas. Indeed, their own figure from the study indicates that the Pampas was an area with climatic stability. Lumping them together would serve no purpose. Moreover, the genera that inhabited southern South America also tended to inhabit northern and/or central South America or had similar counterparts there2, so the decision to hone in on the former is peculiar.
Critically, if South American extinctions were driven mainly by man, as the research suggests, then this has implications for North America which was settled at the same time by the same group of people (known as Paleo-Indians) 3. We know for a fact that early humans in North America were associated with big-game hunting (i.e. the Clovis)4. As such, it is unlikely that humans played a significantly weaker role in North America than South America. The researchers, to their credit, do mention the sharp difference in extinction intensity between Eurasia and North America despite the former only having a slightly lower climatic footprint, as well as other studies which concluded climate was not the main driver of North American extinctions except perhaps at high latitudes. But this would mean that both North and South America suffered from mostly anthropogenically-caused losses.
It is worth noting that Australia was excluded from the analysis because the extinctions there occurred prior to the time periods selected to measure the footprint of climate changes in the study. However, if it had been included, it would join South America in being a continent with a low climatic footprint but very high extinction rate, meaning there would be two major outliers that cast doubt on the fundamental hypothesis of the researchers. Further, when combined with the fact that North America should follow a similar pattern to South America with regard to human versus climatic causes as mentioned above, this means that extinctions in three of five continents largely cannot be explained by climate, and the alleged link between climate footprint and extinction falls apart entirely.
Although this study is important to highlight as a good example of a study with major flaws, its authors still appear nuanced and well-intentioned. There is no obvious reason to suspect bias, which is more than can be said for some of the other studies that will be examined here.
Case Study 2: Hall’s Cave
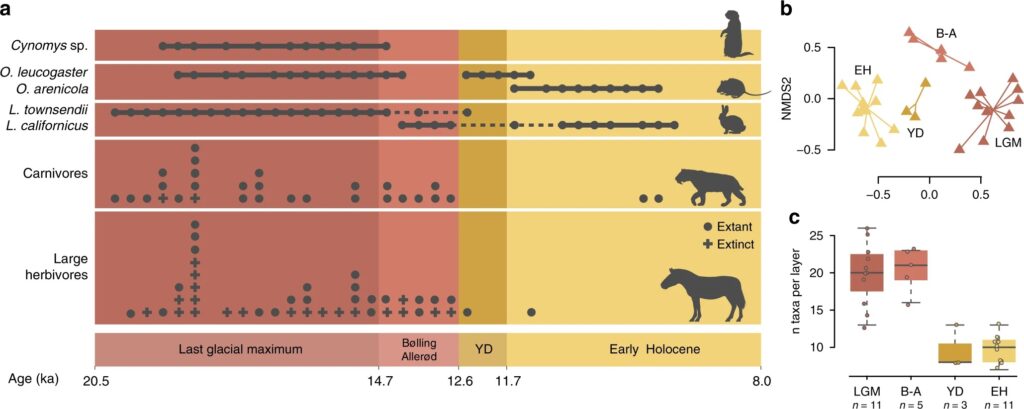
A 2020 study “Rapid range shifts and megafaunal extinctions associated with late Pleistocene climate change” examined fossil plant and animal remains from Hall’s Cave in the Edwards plateau of Texas to analyze paleoecological dynamics between the Last Glacial Maximum and early Holocene5, an interval which includes the window for megafaunal extinctions in North America. The study found dramatic shifts in flora and fauna in response to temperature and moisture changes. More significantly, it revealed that megafaunal, small animal, and plant diversity thrived during the Last Glacial Maximum (LGM) as well as the warm interstadial period known as the Bølling–Allerød (BA), but then declined markedly during the Younger Dryas (YD). The Early Holocene featured a partial recovery of plants and small animals but the megafauna were absent. The study concludes that rapid cooling associated with the Younger Dryas as well as human pressure were causal agents of extinction in the region.
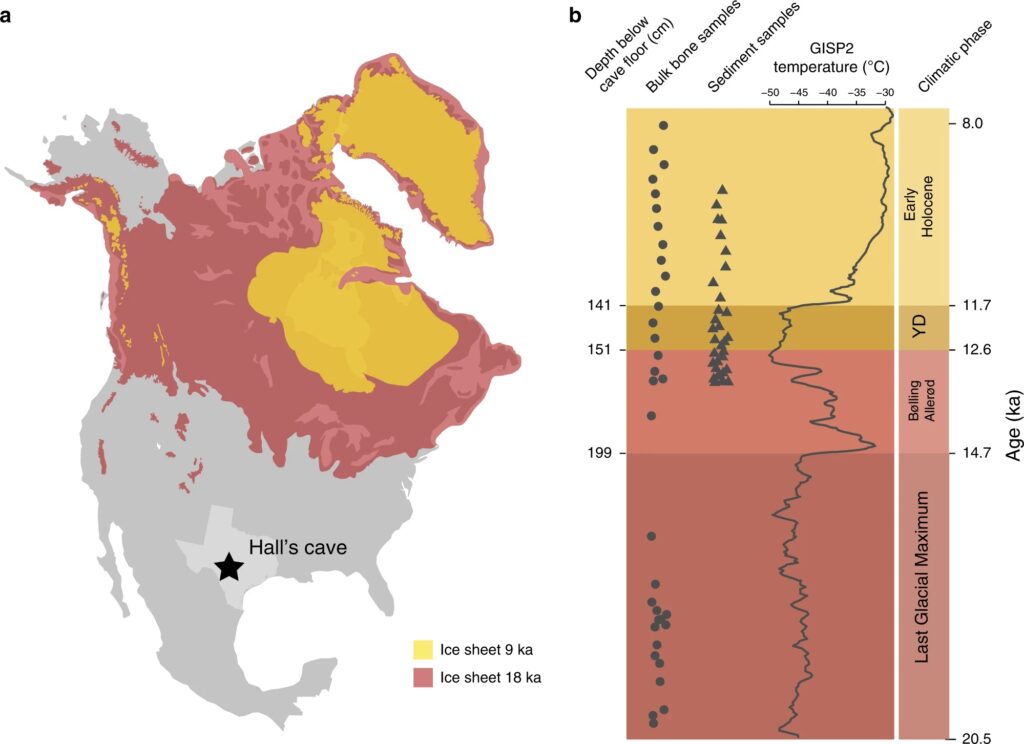
There is much to say about this. For one, it does appear that megafaunal declines coincided to a degree with the Younger Dryas event. However, there is a confounding factor here in that the earlier portion of the Younger Dryas is roughly coincident with the end of the Clovis period, which started just slightly earlier6. The Hall’s Cave study explores and acknowledges a role for humans but fails to explicitly mention the emergence and timing of the big-game hunting Clovis culture, which means they left out crucial context.
Second, the authors do not make a convincing case for why the Younger Dryas specifically was so harmful to the megafauna of this area. This was a cold event which caused a partial return to glacial conditions, but why would a return to glacial conditions be so bad for “ice age” animals? Indeed, the study itself reveals that biodiversity in the region was high during the Last Glacial Maximum (LGM). The authors do mention the speed of change associated with the Younger Dryas, which could offer a contrast between the YD and LGM and explain why biodiversity was so different between the two periods.
But there is an issue: if the problem was not cold conditions but the velocity of change, then the Bølling–Allerød period which preceded the Younger Dryas should have been quite devastating as well. It too featured a similarly sharp change in temperature, albeit in the opposite direction7. In fact, interglacial warmth was achieved during the BA in the Northern Hemisphere8. Yet, the evidence clearly indicates that plant and animal diversity was retained in that period, even if shifted towards more warmth-loving forms compared to the LGM. So what we have here is intact plant and animal diversity during a cold stable period (LGM) and a rapid warming period (BA), followed by a large scale loss of diversity—including the megafauna—during a rapid cooling period (the YD), and a partial recovery during a stable warm period (Early Holocene).
This is a rather odd pattern, but to make matters even more interesting, the “LGM” designation in the study is too inclusive and partially covers a distinct period known as Heinrich Stadial 1, or Oldest Dryas, lasting from around 18-15 thousand years ago. It was more similar to the Younger Dryas than the LGM in its forcings, most importantly as it included a weakening of the thermohaline circulation due to Atlantic freshwater influx7 9. Paleoclimate simulations indicate that temperature seasonality (range in temperature between summer and winter) was similar between the Oldest and Younger Dryas, despite the former being colder10. This may not make much sense to the layman who is unfamiliar with paleoclimatology, but the key takeaway here is that a Younger Dryas-like phase already occurred right between the supposedly ideal LGM and Bølling–Allerød periods, and the megafauna thrived during it.
Therefore, there is no reason why the Younger Dryas itself should feature a unique and severe depletion of diversity if climate is considered such a major culprit. The authors themselves only weakly attempt to explain this. They do acknowledge the possibility of a human role in the disappearance of the megafauna but it is strongly suggested that the extinctions could not have happened without the added stress of climate—the so-called “one-two punch” described in the study. Additionally, there is no mention of humans in the title while the reference to a human contribution in the abstract was only added after peer-reviewers suggested the authors do so, which indicates that while human involvement was ultimately acknowledged, it was done so only begrudgingly.
However, the most concerning aspect of the study is its aforementioned omission of any mention of the Clovis. The fact that this big-game hunting culture emerged and spread rapidly right on the eve of the Younger Dryas has very strong implications with regard to extinction causes, especially since it is hard to argue that climate changes during the Younger Dryas could have been that decisive. The decision to leave out any mention of Clovis, along with the apparent reluctance to explicitly acknowledge a human role upfront (in the title, and initially even in the abstract), means we must question the motivations of the researchers involved—it may be that they were concerned that presenting this important information could undermine their own conclusions about the prominence of climate in extinctions here.
Case Study 3: What Happened to the Woolly Mammoths?
A disproportionate amount of focus when it comes to the Late Quaternary extinctions revolves around the fate of the woolly mammoth (mammuthus primigenius)—the undisputed icon of the ice age. Considerable focus has also been directed towards the animals that coexisted with the woolly mammoths such as woolly rhinos, steppe bison, cave lions, as well as the loss of their principal habitat: the mammoth steppe. The mammoth steppe was a unique biome, containing a mix of steppe and tundra plant and animal species and having high enough productivity to support a large biomass of grazers despite the cold climate11 12.
The disappearance of the mammoth steppe and the extinction or contraction of many of its species is a complex topic, and there does seem to be a better argument for a meaningful climatic role here than in other parts of the world. As the planet became warmer with deglaciation, cold-adapted animals were forced to moved north. Unfortunately, a transition from the graminoid and forb dominated mammoth steppe to a more shrubby type of tundra and boreal forest occurred across most of northern Eurasia and North America.

This shift is commonly believed to be the result of widespread increases in moisture at high latitudes11 13 14, possibly combined with higher CO2 levels15. As such, the very Arctic and Subarctic regions that would have served as refugia for the cold-adapted creatures of the mammoth steppe during interglacials became less optimal. However, an alternative interpretation is that the loss of the mammoth steppe was due to humans, as the large herbivores may have maintained the grasslands through trampling and nutrient cycling, and their extinction at the hands of humans allowed woody vegetation to proliferate unfettered12 16.
For many researchers, climate-driven environmental change is sufficient to explain all or most of the decline or extinction of mammoth steppe megafauna. The problem, however, is that the same animals survived multiple past interglacials (albeit with reduced population sizes or even bottlenecks17) only to succumb at the transition to the present one on the Eurasian and North American mainlands. Moreover, in the Holocene, woolly mammoths continued to thrive in two rather small islands in the far north long after they had been eliminated everywhere else, which is rather surprising given the sheer size of the mainlands of Eurasia and North America which surely must have provided some suitable habitat.
A study titled “Life and extinction of megafauna in the ice-age Arctic” was published in 2015 and has since been cited 59 times. It looks at the dynamics of environmental change during the glacial-interglacial transition on the North Slope of Alaska and the effects on the megafauna of that region to determine the causes of extinction18. The study correctly points out that lengthy interstadials were not ideal for grazing megafauna because they culminated in the widespread development of peatlands and shrub vegetation in Beringia, and that major collapses in the populations of Arctic megafauna such as horses, bison, muskoxen, and woolly mammoths, occurred during the last such interstadial (the Bølling–Allerød).
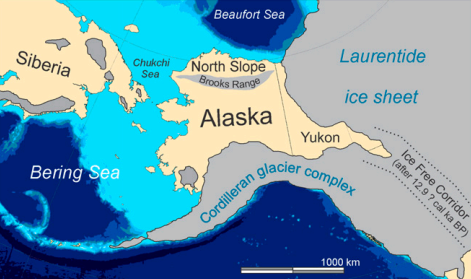
But there are glaring issues in the paper. The researchers hone in entirely on the climatic dimension, on the basis of claiming that the human population in the North Slope region was far too low to have affected the megafauna. They describe two geographical barriers at the time that sealed the fate of the megafauna of eastern Beringia, the flooding of the Bering land bridge and the late and slow opening of the ice-free corridor between the Laurentide and Cordilleran Ice sheets. These two facets prevented the animals from dispersing west into better habitat in northeastern Siberia or south into the continental United States respectively, effectively trapping them:
The end of the last ice age was probably uniquely fatal for arctic megafauna because of the unusual intersection of two events: widespread paludification that drastically reduced range quality for megafaunal grazers and simultaneously hindered their ability to disperse across the resulting soggy landscape and flooding of dispersal routes to Asia before the ice-free corridor leading to lower-latitude North America fully opened.
The most troubling aspect of this paragraph is the use of the term “uniquely fatal” to describe the last glacial–interglacial transition. For this description to be accurate, the authors would need to clarify what made this particular transition so different from earlier ones—many of which saw sea levels rise just as much or even more and also featured a submerged Bering Land Bridge—especially given genetic evidence that woolly mammoths persisted through the previous interglacial in a North American refugium 17. Yet, the authors make no effort whatsoever to confront this problem.
They also fail to mention the fact that woolly mammoths and company in mainland northeast Siberia and in mid-latitude North America (a population existed there as well19) did not survive the extinction event either, meaning that the inability of the animals to disperse to these regions was completely irrelevant to their ultimate demise. The authors dismiss a human contribution for extinctions in the study region, but one is absolutely necessary to explain the loss of these creatures across the far north.
Multiple studies, however, have sought to explain why woolly mammoths and their contemporaries survived earlier interglacials but not the most recent one, the Holocene. One palaeoecological analysis examined last interglacial (Eemian) deposits from Bolshoy Lyakhovksy Island, part of the New Siberian Islands off the Arctic coast of Siberia, and found evidence that it was once covered by steppe-tundra rather than today’s monotonous wet Arctic tundra20. Additionally, herbivores appear to have thrived on the island on the basis of coprophilous fungi spores and possible evidence of vegetational disturbance from grazing pressure.

The authors attributed these drastically different conditions during the previous interglacial compared to the modern-day at the site to a drier climate and more continental climate with warmer summers, resulting from higher summer insolation and lower sea levels that kept the coastline farther away. They argued that the Holocene marine transgression increased oceanic influence across not just that locale but over large parts of Siberia and maybe even Alaska—resulting in cooler summers, warmer winters, and cloudier, wetter conditions compared to the previous interglacial—and that this shift may have contributed to mammoth extinction.
There are problems with this notion, however. The Eemian climatic reconstruction for the site as suggested by the researchers—warmest-month temperatures of at least 12.5°C, relatively clear skies, and annual precipitation no higher than 250 mm—is typical for much of interior Siberia today, only farther south in the mainland. Climate data indicates that the two towns in Northern Hemisphere with the coldest winter temperatures, Verkhoyansk and Oymyakon in the Sakha Republic, closely approximate these metrics (as do Inuvik and Kugluktuk in far northern Canada, interestingly). Additionally, if such hyper-continental conditions were critical for mammoth survival, the species should have persisted longest in northeastern Siberia’s interior. Instead, the last surviving populations were confined to two perpetually overcast islands: Wrangel in the Arctic and St. Paul in the Bering Sea.

Admittedly, there may be truth to the notion that suitable habitat for woolly mammoths is somewhat diminished compared to the previous interglacial. The paleoenvironmental research is probably sound. What is not sound, however, is how the researchers decided to dismiss the possibility of a human role in the extinction of mammoth steppe fauna in the most absurd and unscientific way imaginable:
Whereas the mammoth faunal complex and tundra steppe persisted during the former Quaternary warm stages presumably in their Siberian refugia, they largely perished in the Holocene. The overkill theory explains the disappearance of the mammoth faunal complex as the result of human hunting (e.g. Zimov, 2005; Haynes, 2007). After the extermination of mega herbivores, the lack of grazing pressure would accordingly result in a vegetation shift from grassland to tundra. According to the predator-prey equations of Lotka and Volterra, however, it is impossible that a predator extirpates its prey (Volterra, 1931). Palaeolithic and Mesolithic man in high latitudes was entirely dependent on game animals as agriculture was not yet developed and anyway impossible so far north. Humans could not eliminate their basic recourses before becoming extinct themselves.
We already know this is false because we have clear and documented evidence of predators, in this case humans, wiping out species on several islands. As for the idea that early humans at high latitudes could not have eliminated megafauna without going extinct themselves, this is based on the assumption that they could not switch to alternative food sources. We know there had to have been some; hunter-gatherers have inhabited northeast Siberia and the Arctic in general for several millennia during the Holocene, long after the megafauna disappeared.
There was no need to reference an equation from an almost century-old source to “prove” something that is already demonstrably false, i.e. the notion that predators cannot hunt their prey to extinction. While the paleoenvironmental analysis in the study is interesting, inserting this bizarre section for the purpose of dismissing human influence undermines the objectivity of the study. This is particularly disappointing given that the authors—Pavel Tarasov, Frank Kienast, and Andre Andreev—are distinguished paleoecologists who should know better.
Another study from 2013 used complex vegetation models to examine the differences in land cover in northern Eurasia between the last interglacial and the Holocene21. Its simulations showed that herbaceous tundra was somewhat more widespread in Siberia during the last interglacial (120 kya) than during the mid Holocene (6 kya). The authors enthusiastically state that this supports the view that the “particular nature of environmental changes” during the last glacial–interglacial transition were the primary cause of megafaunal extinctions:
Furthermore, the contrast in vegetation characteristics of East Siberia between the last interglacial and the Holocene provides support for the argument that the particular nature of the environmental changes after the last glacial maximum and during the early Holocene was the primary factor in causing the extinctions of many larger vertebrates of Eurasia during that period, rather than the coincidental increase in the geographical range, abundance and/or technological status of anatomically modern humans.
Yet, when one actually compares the projections from this study, it is hard to describe the vegetative differences between the previous interglacial and the current one as anything other than modest. Although slightly decreased compared to the Eemian, Holocene northeast Siberia still shows plenty of areas suitable for herb cover. It is also worth noting that Arctic North America—where mammoths also went extinct— seems essentially indistinguishable.
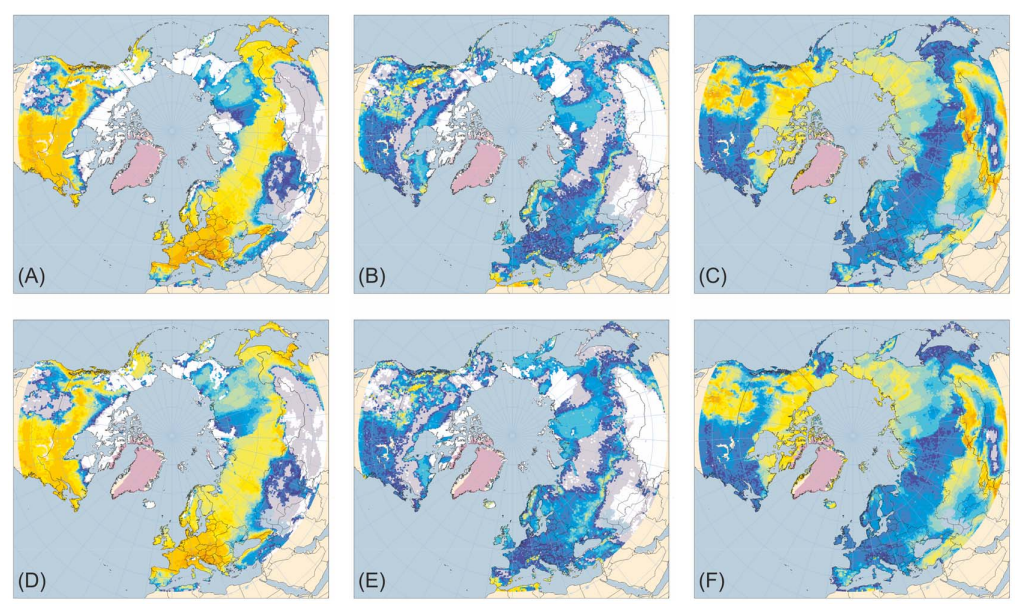
The slight differences in vegetative cover between the previous interglacial and the current one cannot alone explain why woolly mammoths in the former were able to survive the entirety of the latter in three different refugia, as suggested by another study which did genetic analysis on mammoth remains17, while mammoths in more recent times had already gone extinct on the mainland by the Holocene22 23. It is difficult to explain the patterns seen here without invoking an important-but not exclusive-role for human beings in the extinction of mammoth steppe megafauna.
What all of these studies have in common is they fail to account for the sheer ecological breadth of woolly mammoths. It is bizarre to claim that the entirety of the present-day Arctic or Subarctic, including northeastern Siberia, is not dry and/or continental enough for woolly mammoth to inhabit when we know for a fact that during the Pleistocene, the animals thrived in areas which were much less dry and/or continental. For example, woolly mammoths were present during certain intervals in southern Spain, where the climate at the time was dry but relatively mild temperature-wise24 25. They were also present at the Saltville site in southern Virginia, which was moist enough to support marshes and hardwood trees26. Mammoths in southeastern Russia (Primorye) likewise inhabited a fairly humid environment that contained plenty of forest cover27.
A landmark paper came out last year which looked at a different mammoth steppe animal—the woolly rhinoceros—and found that humans were an essential factor in its extinction28. The authors accounted for the wide climatic tolerance of the species to reveal that climate change alone could not have been the cause of its demise. Instead, its extinction was a long process with the cumulative effects of human hunting over tens of millennia being detrimental to its dispersal ability, which combined with climate change to deal a fatal blow. Any believable model incorporating climate into woolly mammoth extinction should resemble the woolly rhino scenario—one in which human impact was a necessary factor alongside climate, rather than climate being the sole cause.
Woolly mammoths, in many ways, serve as a proxy for the debate on extinctions as a whole. As such, the popular tendency to shift all or the almost all of the blame for woolly mammoth extinction onto climate is significant and will be reexamined at the end of this article.
Case Study 4: Convoluted Mechanisms
Climate change in the form of (often rapid) precipitation and temperature shifts is the most frequently cited form of climatic causation for the extinction of megafauna during the Late Quaternary. Indeed, it is not surprising considering that the terminal Pleistocene was a time of profound and rapid change. But a few papers have hypothesized about unconventional climate-related mechanisms, different from the typical view involving profound changes in temperature or precipitation.
One such paper by J.T. Faith suggests that the large increase in CO2 during the last glacial-to-interglacial transition may have been a culprit29. The author correctly points out that changes in many metrics that influence plant nutrition such as precipitation and temperature were varied greatly across time and geography during the transition, and that the only consistent global change was the increase in atmospheric CO2. He posits that this increase may have been decisive for the collapse of megafaunal populations in North America because higher CO2 leads to greater plant biomass but a lower density of nutrients within that forage (as nutrients in any given environment are limited).
This less nutritious biomass then led to nutrient stress among megafauna and, possibly in concert with other forces, caused their populations to collapse. The author uses the allegedly earlier sporormiella collapse in eastern North America compared to western North America as evidence for this theory, because the greater moisture in the east would have combined with the increasing CO2 to cause ecosystems to reach this “nutrient deficient” state sooner.
Yet, there are major problems with this idea. Firstly, the onset of widespread extinctions in North America south of the ice sheets took place close to the Bølling–Allerød/Younger Dryas boundary30, when CO2 levels were around 240 ppm7. While this is not far from interglacial quantities (around 260-280 ppm), it was still only about 55 ppm higher than the LGM (~185 ppm) and perhaps only 40 ppm above typical stadial conditions (~200 ppm). Moreover, this increase in CO2 took place over thousands of years. In modern times, we have witnessed a 140+ppm increase in CO2 concentrations compared to pre-industrial levels over the course of a mere two centuries, and there has been no mass die-off of megafauna in protected areas where they still exist in large numbers such as Yellowstone, Kruger, or Kaziranga.
If some arbitrary nutrient threshold had been crossed 13-12 thousand years ago with relatively modest and slow increases in CO2 at the time triggering a megafaunal collapse, the same should have happened several times over in the last few centuries. This is especially so considering that the capacity for nutrient transport at the present is far lower than during the Pleistocene31. Moreover, evidence from sporormiella concentrations suggests that animal populations in Kenya—where wildlife had already adapted to human presence—remained stable or even grew during the climate transition32, which raises the question of why elevated CO2 levels would have posed a unique problem for North America.

This is of course not at all the most popular climate-related theory for what caused the extinctions in North America. Yet, it has faced essentially no rebuttals and been cited 40 times. Given how flimsy the basis for this hypothesis is, this is quite remarkable and suggests that proper skepticism is not being applied when it comes to climatic explanations.
Another paper titled “Climate-driven ecological stability as a globally shared cause of Late Quaternary megafaunal extinctions: the Plaids and Stripes Hypothesis” came out in 2019, and takes the unusual stance of pinning a large portion of the blame not on climatic instability, but (shockingly) on climatic stability33. The premise is based on the idea that the glacial period, with its centennial and millennial-scale climate shifts, was better for megafauna than the more stable Holocene (11,700 years ago to present). The rapid changes in climate combined with a lag in vegetative changes meant that it was a world where floral composition was always in a state of transition as opposed to reaching an equilibrium with climate.
In contrast to the “striped” pattern of Holocene ecology where floral and faunal characteristics are dependent on geography, the Pleistocene ecology featured a “plaid” pattern where the perpetually changing climate added an extra layer of complexity—complexity which allegedly benefitted large animals. The authors attempt to reconcile this interpretation of instability being preferable to stability by speculating that a large portion of the animals that appear—due to the Signor-Lipps effect—to have gone extinct at the end of the Pleistocene actually went extinct in the Holocene, and that the Holocene may be longer and more stable than previous interglacials. According to them, Earth’s ecosystems shifting from a “plaid Pleistocene” to a “striped Holocene” pattern, along with other factors (such as humans) exerting their own pressures, could have been what caused so many animals to go extinct.
Here we hypothesize that one of the most important
of these underlying, shared causes of Late Quaternary
extinctions was a fundamental shift in the spatio-temporal
‘fabric’ of ecosystems that occurred during the ice
age/Holocene transition. Rapidly changing climate during
the ice age created widespread transient ecosystems in which
the eco-physiological traits associated with large body size
endowed megafaunal species with special advantages. But
these and other traits associated with being large became
disadvantages within the more stable climate regime of
the Holocene and thus lowered the extinction thresholds
for many megafaunal species. Once these thresholds were
lowered, individual species fell victim to a variety of
proximate extinction causes, whether overkill by humans,
disease, habitat loss, or disrupted trophic cascades.
The issue with this paper is obvious. While we know for certain that some extinct Pleistocene megafauna technically made it into the Holocene, their abrupt declines undeniably predate it on each continent. Even in North America and South America where megafaunal collapse was most proximate to the Holocene, all evidence—on the basis of fossils and sporormiella concentrations— indicates that it actually began during the glacial-interglacial transition30 34 35 36, not later. In fact, the very authors of this study were responsible for the Alaskan North Slope study a few years earlier which decidedly indicated that declines of Alaskan megafauna occurred during the glacial-interglacial transition, and specifically during the Bølling–Allerød18.
In mid-latitude North America, the climate was changing so rapidly during the Pleistocene-to-Holocene transition that it resulted in the widespread presence of floral communities that have no modern analogues37. This is problematic for the hypothesis—it does not get any more “plaid” than that. The authors failure to accept the inconvenient fact that animal communities had already collapsed in the New World by the start of relatively stable Holocene conditions, even if some extinct species technically survived into the epoch, means that their entire hypothesis is built on a false premise.
The authors invoke the length of the Holocene interglacial in comparison to previous ones as proof for their claim, suggesting that extinctions would have been less severe if it had been shorter. It is certainly true that the current interglacial has been rather long and is in fact set to continue for a much longer period of time38. Yet, the mere fact that (continental) extinctions occurred prior to the Holocene, and may have at best bled into the very beginning of it, means that this fact is completely irrelevant to extinction dynamics.
Just like the nutrient paper by J.T. Faith, this is only a hypothesis and the authors do concede that ecological stability may have been just one cause within a “hierarchy” of causes for the Late Quaternary Extinctions. Still, it entirely contradicts the available evidence regardless of whether it is placed at the top or the bottom of this hierarchy. Moreover, there is a point to highlighting these two studies: new hypotheses or models are usually borne out of a need to explain gaps in knowledge that cannot be explained well by prevailing theories, but extinctions in the New World during the glacial-interglacial transition are already smoothly explained by human agency.
Yet, if researchers are coming up with new, implausible, and frankly eccentric ways to force climate into a phenomenon that is more convincingly human-driven, it begs the question of what the authors are actually hoping to accomplish. Are they truly just following the evidence to where it leads, or is there something else at play? This will be the focus of our next section.
Climate Favoritism
To recap, the studies analyzed here all have major weaknesses. And unlike studies that promote anthropogenic explanations, which themselves may contain some inaccuracies, the weaknesses in these climate-centered studies are so severe that they practically invalidate the conclusions drawn by the researchers. In just the few examples covered here, we have seen strained and unscientific efforts to reconcile climate-centric hypotheses for the Late Quaternary Extinctions with data that clearly contradicts them. A human role is frequently unfairly dismissed and at other times only begrudgingly acknowledged. Crucial information is routinely omitted, and the lengths to which some researchers go to inflate the role of climate while minimizing that of humans are so profound that they raise legitimate questions about bias.
In the preceding section, we looked at two unconventional hypotheses concerning the Late Quaternary Extinctions: one proposed by J.T. Faith, attributing megafaunal collapse in North America to rising CO₂ levels, and another by a separate group of scientists suggesting that the climatic stability of the Holocene was a major driver of extinctions. In both cases, the evidence presented was unimpressive and there is already a much more convincing scenario readily available in the form of (mainly) anthropogenic causation. Why then would these authors feel the need to promote these peculiar and improbable ideas?
This makes perfect sense if we consider that the scientists behind these papers are inclined toward climatic explanations and intent on diminishing the human role. They likely recognize, at least on a deeper level, the shortcomings of traditional climate-based models but remain unwilling to accept the main alternative to them (predominantly human causation). As such, they feel the need to “shop” for novel climate-based hypotheses to attempt to bolster the climatic contribution in any way possible—regardless of whether they withstand scrutiny or are consistent.
A more concerning problem is the presence of what appears to be, at best, inconsistency and, at worst, clear contradictions among the scientists. As mentioned earlier, the authors of the “Plaids and Stripes hypothesis” paper had previously published a study asserting that megafauna in the North American Arctic went extinct before the Holocene, even rejecting claims from another study which, based on sedimentary DNA, claimed survival of horses and mammoths as late as 7,500 years before present. Yet four years later, they published the “Plaids and Stripes” study which instead speculates that a large swathe of “late glacial” extinctions possibly occurred during the Holocene and were caused by interglacial stability. Data indicates that extinctions in mainland North America did not take place much later than in the Arctic regions either39, so this is clearly not a case of scientists updating their views in light of new data. It is an example of stretching the limits of plausibility to fit a convenient narrative.
Furthermore, one of the “Plaids and Stripes” authors—Beth Shapiro—was also a coauthor of another paper critiqued above, the Hall’s Cave study, which dated megafaunal extinctions on the Edwards Plateau of Texas to the Younger Dryas (again, not the Holocene). Looking at the respective dates of publication for the papers—the North Slope study, Plaids and Stripes, and Hall’s Cave—reveals major inconsistency: Shapiro initially attributed North American megafaunal extinctions to pre-Holocene climate shifts, then speculated that stable Holocene conditions may have been a culprit, and then once again blamed rapid pre-Holocene climate change. This blatant contradiction and inability to commit to a coherent narrative with regard to climate-based explanations should raise eyebrows.
Continuing this pattern, Shapiro’s book How to Clone a Mammoth: the Science of De-extinction advocates the creation of a woolly mammoth proxy, created by genetically modifying an Asian elephant, which would then be released into the wild somewhere in the Arctic40. While she acknowledges that true de-extinction is impossible, she argues that such an animal could help “restore lost productivity” to Arctic ecosystems. Yet if we take her other claims at face value—that the Holocene climate was too warm, wet, or stable for mammoths to survive—then her dedication to releasing mammoth proxies into these same environments appears puzzling. Why promote the reintroduction of a species to an ecosystem that she has characterized as suboptimal for it?

We are therefore faced with two contradictions: first, the inconsistency between Shapiro’s own extinction hypotheses and second, the disconnect between her research conclusions regarding the demise of woolly mammoths and her advocacy for mammoth de-extinction. These contradictions are difficult to explain without considering the possibility of an underlying bias. The Plaids and Stripes paper’s bizarre claim—that climatic stability rather than instability caused extinctions—directly conflicts with her prior and subsequent work, suggesting that the main goal is not to reach some sort of truth on the matter but to bolster the significance of climate in extinction dynamics by any means possible, even if by invoking opposing mechanisms. Similarly, her support for mammoth de-extinction implies that she may privately regard Holocene conditions as less unsuitable than her public research has asserted.
For the record, this is not an obscure scientist; Beth is one of the best-known names in paleontology. She is the lead scientist for the company known as Colossal Biosciences, which earlier this year was at the center of a massive controversy involving their (supposed, not actual) de-extinction of the dire wolf. Like many within the scientific community, she appears to exhibit a consistent bias in favor of climatic explanations over anthropogenic ones—a tendency that warrants closer scrutiny of the motivations and ideological drivers that may be at its root.
Possible Motivations for Bias
It is understandable that many want to emphasize the importance of climate change in the Late Quaternary Extinctions, as most of the extinctions took place during the Pleistocene when the Earth was frequently subject to profound climatic upheavals. Yet, some researchers have even attempted to bring climate in to explain extinctions during the relatively stable Holocene. For example, a few researchers have argued that an intense drought was at least partially responsible for the extinction of Madagascar’s megafauna during the Late Holocene41 42 43. Although other scientists have firmly refuted this particular notion44 45, it fits into a broader pattern along with the rest of the evidence presented in this article indicating that a large segment of scientists have a strong inclination to inflate the role of climate change in faunal extinctions. But why?
The most obvious reason for why scientists may feel the need to bolster the significance of climate change is to downplay the human role. The question of whether mankind eliminated the megafauna is more than it appears at face value; it is ultimately also a question about what it fundamentally means to be human. The notion that extinctions were inevitable when prehistoric peoples crossed paths with megafauna on new continents is not a pleasant one and has the potential to induce guilt. That guilt can be compounded with already existing guilt over other aspects of human behavior and actions both in the present and in (recorded) history to become overwhelming.
There is a widely accepted (especially in academia) but fanciful notion that pre-industrial and especially pre-agricultural societies lived in perfect harmony with nature, only taking as much as they needed. This is known as the “noble savage” trope; the book “Constant Battles: Why We Fight” by Steven LeBlanc dives deep into why this concept is deeply incorrect46. The notion of hunter-gatherer societies delivering widespread ecological damage, as would be the case if we accept a mostly anthropogenic cause for the LQE, is therefore a direct contradiction of this popular theory.
But the evidence for at least some sort of human involvement in the LQE is overwhelming, which means that positing purely climatic causes for them is incredibly challenging, at least globally. Some researchers have sought to deny human agency altogether in various continents but it is a much more common tactic to frame humans as having a role but being roughly as important or less important than climate in extinction as opposed to being central to it. In other words, minimization.
There is a lot of solace in this line of thinking. Promoting the notion that “humans were not capable of delivering megafaunal collapse on their own and something else was needed” eliminates the apparent inevitability of extinction occurring when critical population density and technology thresholds are crossed following human settlement. In doing so, we can feel less guilty, and not really have to question the fanciful notion that people lived in perfect harmony with nature. It only needs to be slightly modified: “Yes humans can and do live in peace with nature, and widespread extinction is not destined when they discover new continents; it only happens when the environment is changing rapidly”. It makes us feel a little better about being human generally. But there is another, related dimension.
The most severe extinctions took place in the Americas and Oceania. As far as we can tell, the human groups responsible for extinctions in these areas are the direct ancestors of the indigenous inhabitants of those areas; Native Americans, Australian Aboriginals, and Papuans. These indigenous peoples have faced historical oppression and disenfranchisement. The noble savage trope has been strongly applied to various native groups, who are frequently portrayed as stewards of the environment, in contrast to European colonizers who are considered to have wreaked colossal damage on local ecosystems.

However, acknowledging that the first settlers of these continents themselves wiped out a vast number of species throws a wrench into this. Some scientists may seek to “shield” indigenous groups from potential blame and demonization for early extinctions given the injustices they have already faced in recent history, as well as to maintain ideas about peaceful coexistence between said groups and their local flora and fauna. Therefore, we can speculate there is an important historical component involved in the promotion of climate-heavy theories for the LQE. But there is still another aspect to consider.
We are currently experiencing rapid climate change due to greenhouse gas emissions. Studying the effects of past climate changes on animals has therefore become a priority as we simultaneously try to mitigate anthropogenic climate change and attempt to predict how it may affect ecosystems. For scientists studying the topic, they can demonstrate real world relevance for their research by attributing climate-heavy causes to the LQE. This creates a sense of purpose and value in the research, in stark contrast to arguing that climate was greatly subservient to early human activity in extinction. The latter would only induce feelings of guilt in many.
As I said earlier in the article, the debate around the fate of woolly mammoths is something of a proxy for the debate on extinctions as a whole. It is true that the disproportionate research into them is driven simply by the fact that they are the most iconic animals of the ice age, but in light of everything we have discussed, we may speculate on whether there may be another dimension to this hyper-fixation on them (and other animals that shared their habitat). Why would some researchers be so determined to declare a wholly or mostly climatic role for their demise despite strong reason to believe that humans played an essential part as well?

The human population in Arctic and Subarctic climate zones, where woolly mammoths lived, has always been very low—certainly lower than in more temperate and tropical zones47. And there was indeed a widespread and dramatic shift at the end of the Pleistocene in the far north from rich forb and graminoid tundra to less palatable shrub and moss tundra, whereas productive habitats, including grasslands, were retained at temperate and tropical zones. Therefore, making the case for a climate-dominant pathway for extinction of far northern megafauna is much easier than for their mid and lower latitude counterparts.
However, if climate cannot explain all or most of the extinction of megafauna in Arctic and Subarctic climate zones where the human population was small and environmental change was profound (steppe-tundra–>shrub tundra and taiga), then it becomes much harder to justify a substantial role for climate change in the LQE elsewhere in the world where human populations were denser and environmental changes were far more benign. Hypothetically, if we were to concede that extinctions in the mammoth steppe were only about 50% climate change related and the rest anthropogenic, then extinctions in South America, for example, would probably have to be less than 10% climatic and 90% or more anthropogenic. Avoiding that logical, but unsavory conclusion could be a subconscious motivation to dismiss the human role in Arctic/Subarctic extinctions.
The reluctance of so many scientists to grapple with the reality of Late Quaternary Extinctions has had implications beyond academic circles themselves. The average person is well-aware that the extinction of the dinosaurs was the result of an asteroid impact, but how many laymen are able to answer what caused the extinction of mammoths and sabre-toothed cats? During the Colossal Biosciences dire wolf “de-extinction” fiasco earlier this year, it was difficult to find any mainstream news articles which explicitly stated that humans—through competition and other pressures—were responsible for their extinction, despite the widespread coverage of the story.
Over in Australia, a documentary released last year by ABC Science explored the extinction of that continent’s Pleistocene megafauna. The film spends the majority of its airtime describing the alleged phenomenon of unprecedented climate change, specifically increasing aridity. It suggests that the aridification, and hence extinction of the Australian megafauna, was driven heavily by some sort of geomagnetic excursion around 42 thousand years ago, which is actually a fringe theory48 that has not gained acceptance in the scientific literature49 50. Humans are eventually acknowledged, but only toward the end and in reluctant fashion, giving the viewer the impression that humans were only “the straw that broke the camel’s back” as opposed to the principal driver of extinctions there. This pattern of bias closely mirrors that seen in academic discussions of the Late Quaternary Extinctions.
Implications and Conclusion
The bottom line in all of this is that a lot of the scientific papers that emphasize climate-centric models for the LQE are deeply flawed in one way or another and can easily be undermined by critical analysis. A lot of the research is so questionable and contradictory that serious questions about the potential biases of its authors have to be raised. In reality, this is not a battle between equally plausible ideas and we cannot use some sort of “argument to moderation” in order to understand the Late Quaternary extinctions, even though the literature seems roughly evenly split regarding the importance of causes51. Climate-centered theories only appear credible because proper scrutiny has not been applied to them.
I would like to reiterate that we do not know everything about the Late Quaternary extinctions. There is still a lot that more research can tell us about them. However, hyper-emphasizing the climatic role only creates more questions, which is the opposite of what good science is supposed to do. Meaningful progress in ascertaining the details of the extinctions will mostly come from an honest and deep analysis of the anthropogenic dimension, and this can only be done if the best and brightest minds in paleontology and archeology move beyond the tendency to minimize human agency. Accepting the pivotal role of human beings should therefore become a priority for any scientists genuinely interested in the truth.
Further, I want to make it abundantly clear that I am not arguing that research into how climate change affected past animal communities, including during the Late Quaternary, is not important. It obviously is. However, scientists must study the effects of Late Quaternary climate changes with the understanding that the impact of those changes on fauna were secondary to those of humans.
It is in many ways heartening that there are people in the world with such a passion for paleontology and paleoecology that they have dedicated their lives to furthering our understanding of it. But scientists studying these topics should engage in some more reflection. Those who analyze things such as how glyptodonts and ground sloths in the Americas persisted for tens of millions of years, how hippos colonized England during interglacials52, or how caribou extended their ranges into Spain during glacial periods53 should recognize and admire the remarkable ability of animals to withstand and adapt to major environmental changes.
Portraying Late Quaternary animals as being practically helpless in the face of climatic and environmental changes, as is very often done in the context of discussing their extinctions, betrays a fundamental lack of respect for nature and science itself. This is unbecoming of those who would profess to have a deep love for the natural world and history and study these topics for a living. Scientists would do well to avoid engaging in this sort of behavior and give these bygone creatures the respect they deserve by discussing the causes of their demise honestly.
- Nogués-Bravo, D., Ohlemüller, R., Batra, P., & Araújo, M. B. (2010). Climate Predictors of Late Quaternary Extinctions. Evolution, 64(8), 2442–2449. https://doi.org/10.1111/j.1558-5646.2010.01009.x[↩]
- Cione, A. L., Tonni, E. P., & Soibelzon, L. (2009). Did humans cause the Late Pleistocene-Early Holocene mammalian extinctions in South America in a context of shrinking open areas?. American megafaunal extinctions at the end of the Pleistocene, 125-144.[↩]
- Llamas, B., Fehren-Schmitz, L., Valverde, G., Soubrier, J., Mallick, S., Rohland, N., Nordenfelt, S., Valdiosera, C., Richards, S. M., Rohrlach, A., Romero, M. I. B., Espinoza, I. F., Cagigao, E. T., Jiménez, L. W., Makowski, K., Reyna, I. S. L., Lory, J. M., Torrez, J. A. B., Rivera, M. A., & Burger, R. L. (2016). Ancient mitochondrial DNA provides high-resolution time scale of the peopling of the Americas. Science Advances, 2(4), e1501385. https://doi.org/10.1126/sciadv.1501385[↩]
- Waguespack, N. M., & Surovell, T. A. (2003). Clovis Hunting Strategies, or How to Make out on Plentiful Resources. American Antiquity, 68(2), 333–352. https://doi.org/10.2307/3557083[↩]
- Seersholm, F. V., Werndly, D. J., Grealy, A., Johnson, T., Keenan Early, E. M., Lundelius, E. L., Winsborough, B., Farr, G. E., Toomey, R., Hansen, A. J., Shapiro, B., Waters, M. R., McDonald, G., Linderholm, A., Stafford, T. W., & Bunce, M. (2020). Rapid Range Shifts and Megafaunal Extinctions Associated with Late Pleistocene Climate Change. Nature Communications, 11(1). https://doi.org/10.1038/s41467-020-16502-3[↩]
- Waters, M. R., Stafford, T. W., & Carlson, D. L. (2020). The age of Clovis—13,050 to 12,750 cal yr B.P. Science Advances, 6(43), eaaz0455. https://doi.org/10.1126/sciadv.aaz0455[↩]
- Clark, P. U., Shakun, J. D., Baker, P. A., Bartlein, P. J., Brewer, S., Brook, E., Carlson, A. E., Cheng, H., Kaufman, D. S., Liu, Z., Marchitto, T. M., Mix, A. C., Morrill, C., Otto-Bliesner, B. L., Pahnke, K., Russell, J. M., Whitlock, C., Adkins, J. F., Blois, J. L., & Clark, J. (2012). Global climate evolution during the last deglaciation. Proceedings of the National Academy of Sciences, 109(19), E1134–E1142. https://doi.org/10.1073/pnas.1116619109[↩][↩][↩]
- Denton, G., Alley, R., Comer, G., & Broecker, W. (2005). The role of seasonality in abrupt climate change. Quaternary Science Reviews, 24(10-11), 1159–1182. https://doi.org/10.1016/j.quascirev.2004.12.002[↩]
- Clark, P. U., Pisias, N. G., Stocker, T. F., & Weaver, A. J. (2002). The role of the thermohaline circulation in abrupt climate change. Nature, 415(6874), 863–869. https://doi.org/10.1038/415863a[↩]
- Liu, Z., Carlson, A. E., He, F., Brady, E. C., Otto-Bliesner, B. L., Briegleb, B. P., Wehrenberg, M., Clark, P. U., Wu, S., Cheng, J., Zhang, J., Noone, D., & Zhu, J. (2012). Younger Dryas cooling and the Greenland climate response to CO2. Proceedings of the National Academy of Sciences, 109(28), 11101–11104. https://doi.org/10.1073/pnas.1202183109[↩]
- Dale Guthrie, R. (2001). Origin and causes of the mammoth steppe: a story of cloud cover, woolly mammal tooth pits, buckles, and inside-out Beringia. Quaternary Science Reviews, 20(1-3), 549–574. https://doi.org/10.1016/s0277-3791(00)00099-8[↩][↩]
- Zimov, S. A., Zimov, N. S., Tikhonov, A. N., & Chapin, F. S. (2012). Mammoth steppe: a high-productivity phenomenon. Quaternary Science Reviews, 57, 26–45. https://doi.org/10.1016/j.quascirev.2012.10.005[↩][↩]
- Monteath, A. J., Gaglioti, B. V., Edwards, M. E., & Froese, D. (2021). Late Pleistocene shrub expansion preceded megafauna turnover and extinctions in eastern Beringia. Proceedings of the National Academy of Sciences, 118(52). https://doi.org/10.1073/pnas.2107977118[↩]
- Wang, Y., Pedersen, M.W., Alsos, I.G. et al. Late Quaternary dynamics of Arctic biota from ancient environmental genomics. Nature 600, 86–92 (2021). https://doi.org/10.1038/s41586-021-04016-x[↩]
- Woillez, M.-N. ., Kageyama, M., Krinner, G., de Noblet-Ducoudré, N., Viovy, N., & Mancip, M. (2011). Impact of CO2 and climate on the Last Glacial Maximum vegetation: results from the ORCHIDEE/IPSL models. Climate of the Past, 7(2), 557–577. https://doi.org/10.5194/cp-7-557-2011[↩]
- Zimov, S. A., Zimov, N. S., Chapin, F. S., Iii, & Zimov, S. A. (2012). The Past and Future of the Mammoth Steppe Ecosystem. Paleontology in Ecology and Conservation. https://doi.org/10.1007/978-3-642-25038-5_10[↩]
- Palkopoulou, E., Dalen, L., Lister, A. M., Vartanyan, S., Sablin, M., Sher, A., Edmark, V. N., Brandstrom, M. D., Germonpre, M., Barnes, I., & Thomas, J. A. (2013). Holarctic genetic structure and range dynamics in the woolly mammoth. Proceedings of the Royal Society B: Biological Sciences, 280(1770), 20131910–20131910. https://doi.org/10.1098/rspb.2013.1910[↩][↩][↩]
- Mann, D. H., Groves, P., Reanier, R. E., Gaglioti, B. V., Kunz, M. L., & Shapiro, B. (2015). Life and extinction of megafauna in the ice-age Arctic. Proceedings of the National Academy of Sciences, 112(46), 14301–14306. https://doi.org/10.1073/pnas.1516573112[↩][↩]
- Schwartz-Narbonne, R., Longstaffe, F. J., Kardynal, K. J., Druckenmiller, P., Hobson, K. A., Jass, C. N., Metcalfe, J. Z., & Zazula, G. (2019). Reframing the mammoth steppe: Insights from analysis of isotopic niches. Quaternary Science Reviews, 215, 1–21. https://doi.org/10.1016/j.quascirev.2019.04.025[↩]
- Kienast, F., Tarasov, P., Schirrmeister, L., Grosse, G., & Andreev, A. A. (2008). Continental climate in the East Siberian Arctic during the last interglacial: Implications from palaeobotanical records. Global and Planetary Change, 60(3-4), 535–562. https://doi.org/10.1016/j.gloplacha.2007.07.004[↩]
- Huntley, B., Allen, J. R. M., Collingham, Y. C., Hickler, T., Lister, A. M., Singarayer, J., Stuart, A. J., Sykes, M. T., & Valdes, P. J. (2013). Millennial Climatic Fluctuations Are Key to the Structure of Last Glacial Ecosystems. PLoS ONE, 8(4), e61963. https://doi.org/10.1371/journal.pone.0061963[↩]
- MacDonald, G. M., Beilman, D. W., Kuzmin, Y. V., Orlova, L. A., Kremenetski, K. V., Shapiro, B., Wayne, R. K., & Van Valkenburgh, B. (2012). Pattern of extinction of the woolly mammoth in Beringia. Nature Communications, 3(1). https://doi.org/10.1038/ncomms1881[↩]
- Dehasque, M., Pečnerová, P., Muller, H., Tikhonov, A., Nikolskiy, P., Tsigankova, V. I., Danilov, G. K., Díez-del-Molino, D., Vartanyan, S., Dalén, L., & Lister, A. M. (2021). Combining Bayesian age models and genetics to investigate population dynamics and extinction of the last mammoths in northern Siberia. Quaternary Science Reviews, 259, 106913. https://doi.org/10.1016/j.quascirev.2021.106913[↩]
- Álvarez-Lao, D. J., Kahlke, R.-D., García, N., & Mol, D. (2009). The Padul mammoth finds — On the southernmost record of Mammuthus primigenius in Europe and its southern spread during the Late Pleistocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 278(1-4), 57–70. https://doi.org/10.1016/j.palaeo.2009.04.011[↩]
- García-Alix, A., Delgado Huertas, A., & Martín Suárez, E. (2012). Unravelling the Late Pleistocene habitat of the southernmost woolly mammoths in Europe. Quaternary Science Reviews, 32, 75–85. https://doi.org/10.1016/j.quascirev.2011.11.007[↩]
- SCHUBERT, B. W., & WALLACE, S. C. (2009). Late Pleistocene giant short-faced bears, mammoths, and large carcass scavenging in the Saltville Valley of Virginia, USA. Boreas, 38(3), 482–492. https://doi.org/10.1111/j.1502-3885.2009.00090.x[↩]
- Ma, J., Wang, Y., Gennady Baryshnikov, Drucker, D. G., McGrath, K., Zhang, H., Hervé Bocherens, & Hu, Y. (2021). The Mammuthus-Coelodonta Faunal Complex at its southeastern limit: A biogeochemical paleoecology investigation in Northeast Asia. Quaternary International, 591, 93–106. https://doi.org/10.1016/j.quaint.2020.12.024[↩]
- Fordham, D. A., Brown, S. C., Elisabetta Canteri, Austin, J. J., Lomolino, M. V., Haythorne, S., Armstrong, E., Hervé Bocherens, Manica, A., Rey-Iglesia, A., Carsten Rahbek, Nogués-Bravo, D., & Lorenzen, E. D. (2024). 52,000 years of woolly rhinoceros population dynamics reveal extinction mechanisms. Proceedings of the National Academy of Sciences, 121(24). https://doi.org/10.1073/pnas.2316419121[↩]
- Faith, J. T. (2011). Late Pleistocene climate change, nutrient cycling, and the megafaunal extinctions in North America. Quaternary Science Reviews, 30(13-14), 1675–1680. https://doi.org/10.1016/j.quascirev.2011.03.011[↩]
- Faith, J. T., & Surovell, T. A. (2009). Synchronous extinction of North America’s Pleistocene mammals. Proceedings of the National Academy of Sciences, 106(49), 20641–20645. https://doi.org/10.1073/pnas.0908153106[↩][↩]
- Doughty, C. E., Roman, J., Faurby, S., Wolf, A., Haque, A., Bakker, E. S., Malhi, Y., Dunning, J. B., & Svenning, J.-C. (2015). Global nutrient transport in a world of giants. Proceedings of the National Academy of Sciences, 113(4), 868–873. https://doi.org/10.1073/pnas.1502549112[↩]
- van Geel, B., Gelorini, V., Lyaruu, A., Aptroot, A., Rucina, S., Marchant, R., Damsté, J. S. S., & Verschuren, D. (2011). Diversity and ecology of tropical African fungal spores from a 25,000-year palaeoenvironmental record in southeastern Kenya. Review of Palaeobotany and Palynology, 164(3-4), 174–190. https://doi.org/10.1016/j.revpalbo.2011.01.002[↩]
- Mann, D. H., Groves, P., Gaglioti, B. V., & Shapiro, B. A. (2018). Climate-driven ecological stability as a globally shared cause of Late Quaternary megafaunal extinctions: the Plaids and Stripes Hypothesis. Biological Reviews, 94(1), 328–352. https://doi.org/10.1111/brv.12456[↩]
- Prates, L., & Perez, S. I. (2021). Late Pleistocene South American megafaunal extinctions associated with rise of Fishtail points and human population. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-22506-4[↩]
- Perrotti, A. G. (2018). Pollen and Sporormiella evidence for terminal Pleistocene vegetation change and megafaunal extinction at Page-Ladson, Florida. Quaternary International, 466, 256–268. https://doi.org/10.1016/j.quaint.2017.10.015[↩]
- Raczka, M. F., Mosblech, N. A., Giosan, L., Valencia, B. G., Folcik, A. M., Kingston, M., Baskin, S., & Bush, M. B. (2019). A human role in Andean megafaunal extinction? Quaternary Science Reviews, 205, 154–165. https://doi.org/10.1016/j.quascirev.2018.12.005[↩]
- Knight, C. A., Blois, J. L., Blonder, B., Macias-Fauria, M., Ordonez, A., & Jens-Christian Svenning. (2019). Community Assembly and Climate Mismatch in Late Quaternary Eastern North American Pollen Assemblages. The American Naturalist, 195(2), 166–180. https://doi.org/10.1086/706340[↩]
- Ganopolski, A., Winkelmann, R., & Schellnhuber, H. J. (2016). Critical insolation-CO2 Relation for Diagnosing past and Future Glacial Inception. Nature, 529(7585), 200–203. https://doi.org/10.1038/nature16494[↩]
- Surovell, T. A., Pelton, S. R., Anderson-Sprecher, R., & Myers, A. D. (2015). Test of Martin’s overkill hypothesis using radiocarbon dates on extinct megafauna. Proceedings of the National Academy of Sciences, 113(4), 886–891. https://doi.org/10.1073/pnas.1504020112[↩]
- Shapiro, B. (2020). HOW TO CLONE A MAMMOTH : the Science of De -extinction. Princeton University Press.[↩]
- Mahe, J., & Sourdat, M. (1972). Sur l’extinction des Vertebres subfossiles et l’aridification du climat dans le Sud-Ouest de Madagascar; description des gisements, Datations absolues. Bulletin de La Société Géologique de France, S7-XIV(1-5), 295–309. https://doi.org/10.2113/gssgfbull.s7-xiv.1-5.295[↩]
- Virah-Sawmy, M., Willis, K. J., & Gillson, L. (2010). Evidence for drought and forest declines during the recent megafaunal extinctions in Madagascar. Journal of Biogeography, 37(3), 506–519. https://doi.org/10.1111/j.1365-2699.2009.02203.x[↩]
- Li, H., Sinha, A., André, A. A., Spötl, C., Vonhof, H. B., Meunier, A., Kathayat, G., Duan, P., Voarintsoa, N. R. G., Ning, Y., Biswas, J., Hu, P., Li, X., Sha, L., Zhao, J., Edwards, R. L., & Cheng, H. (2020). A multimillennial climatic context for the megafaunal extinctions in Madagascar and Mascarene Islands. Science Advances, 6(42), eabb2459. https://doi.org/10.1126/sciadv.abb2459[↩]
- Crowley, B. E., Godfrey, L. R., Bankoff, R. J., Perry, G. H., Culleton, B. J., Kennett, D. J., Sutherland, M. R., Samonds, K. E., & Burney, D. A. (2016). Island-wide aridity did not trigger recent megafaunal extinctions in Madagascar. Ecography, 40(8), 901–912. https://doi.org/10.1111/ecog.02376[↩]
- Godfrey, L. R., Klukkert, Z. S., Crowley, B. E., Dawson, R. R., Faina, P., Freed, B. Z., Hekkala, E., Borgerson, C., Rasolonjatovo, H. A. M., Wright, P. C., & Burns, S. J. (2025). Patterns of late Holocene and historical extinctions on Madagascar. Cambridge Prisms: Extinction, 3. https://doi.org/10.1017/ext.2024.19[↩]
- Leblanc, S. A., & Register, K. E. (2004). Constant battles : why we fight. St. Martin’s.[↩]
- Svenning, J.-C., Lemoine, R. T., Bergman, J., Buitenwerf, R., Roux, E. L., Lundgren, E., Mungi, N., & Pedersen, R. Ø. (2024). The late-Quaternary megafauna extinctions: Patterns, causes, ecological consequences and implications for ecosystem management in the Anthropocene. Cambridge Prisms: Extinction, 2, e5. https://doi.org/10.1017/ext.2024.4[↩]
- Cooper, A., Turney, C. S. M., Palmer, J., Hogg, A., McGlone, M., Wilmshurst, J., Lorrey, A. M., Heaton, T. J., Russell, J. M., McCracken, K., Anet, J. G., Rozanov, E., Friedel, M., Suter, I., Peter, T., Muscheler, R., Adolphi, F., Dosseto, A., Faith, J. T., & Fenwick, P. (2021). A global environmental crisis 42,000 years ago. Science, 371(6531), 811–818. https://doi.org/10.1126/science.abb8677[↩]
- Hawks, J. (2021). Comment on “A global environmental crisis 42,000 years ago.” Science, 374(6570). https://doi.org/10.1126/science.abh1878[↩]
- Picin, A., Stefano Benazzi, Blasco, R., Mateja Hajdinjak, Helgen, K. M., Jean-Jacques Hublin, Rosell, J., Skoglund, P., Stringer, C., & Talamo, S. (2021). Comment on “A global environmental crisis 42,000 years ago.” Science, 374(6570). https://doi.org/10.1126/science.abi8330[↩]
- Stewart, M., Peters, C., Ziegler, M. J., Carleton, W. C., Roberts, P., Boivin, N., & Groucutt, H. S. (2025). The state of the late Quaternary megafauna extinction debate: a systematic review and analysis. Frontiers in Mammal Science, 4. https://doi.org/10.3389/fmamm.2025.1678231[↩]
- Schreve, D. C. (2009). A new record of Pleistocene hippopotamus from River Severn terrace deposits, Gloucester, UK—palaeoenvironmental setting and stratigraphical significance. Proceedings of the Geologists’ Association, 120(1), 58–64. https://doi.org/10.1016/j.pgeola.2009.03.003[↩]
- van, Lazagabaster, I. A., García-Medrano, P., & Cáceres, I. (2025). Southernmost Eurasian Record of Reindeer (Rangifer) in MIS 8 at Galería (Atapuerca, Spain): Evidence of Progressive Southern Expansion of Glacial Fauna Across Climatic Cycles. Quaternary, 8(3), 43. https://doi.org/10.3390/quat8030043[↩]